Valnemulin HCl CAS NO 133868-46-9 Inquire about Valnemulin HCl
Tecoland supplies Valnemulin HCl bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Valnemulin HCl is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Valnemulin HCl 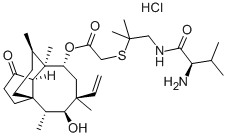
Valnemulin hydrochloride is a new generation of pleuromutilin semi-synthetic antibiotics, which belongs to the diterpene class and belongs to the same class of drugs as tiamulin, and is a special antibiotic for animals. It is mainly used to prevent mycoplasma disease and Gram-positive bacterial infection in pigs, cattle, sheep and poultry. In 1999, it was approved by the European Community for the prevention and treatment of swine dysentery caused by Brachyspira hyodysenteriae infection and swine enzootic pneumonia caused by Mycoplasma pneumoniae infection. It is the first European-wide approved veterinary drug premix, listed as a prescription drug for veterinary use. Approved by the European Community in January 2004 for the prevention of porcine colic spirochetosis caused by infection with Brachyspira coliformis – a mild form of diarrhea in growing pigs and for the treatment of porcine proliferative intestinal infection caused by Lawsonia intracellulare sick. B. hyodysenteriae, B. coli pilus and Lawsonia intracellularis are responsible for most enteritis in growing swine.
| CAS Number: | 133868-46-9 |
|---|---|
| Molecular Formula: | C31H52N2O5S •HCl |
| Molecular Weight: | 601.27 |
| Mechanism of Action: | Valnemulin inhibits protein synthesis in bacteria by binding to the peptidyl transferase component of the 50S subunit of ribosomes. |
| Storage Conditions: | 2-8°C |
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.