Tilmicosin Phosphate CAS NO 137330-13-3 Inquire about Tilmicosin Phosphate
Tecoland supplies Tilmicosin Phosphate bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Tilmicosin Phosphate is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Tilmicosin Phosphate 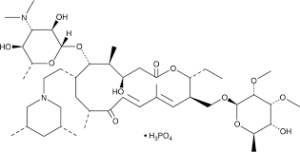
Tilmicosin is a macrolide antibiotic. It is used in veterinary medicine for the treatment of bovine respiratory disease and ovine respiratory disease associated with Mannheimia haemolytica.
Tilmicosin is a macrolide antibiotic was prepared by chemical modifications of desmycosin, and is used in veterinary. It is recommended for treatment and prevention of pneumonia in cattle, sheep and pigs, associated with Pasteurella haemolytica, P. multocida, Actinobacillus pleuropneumoniae, mycoplasma species and other microorganisms found sensitive to this compound. Tilmicosin belongs to 16-membered ring group of class macrolides. The antimicrobial mechanism seems to be the same for all of the macrolides. They interfere with protein synthesis by reversibly binding to the 50S subunit of the ribosome. They appear to bind at the donor site, thus preventing the translocation necessary to keep the peptide chain growing. The effect is essentially confined to rapidly dividing bacteria and mycoplasmas. Macrolides are regarded as being bacteriostatic but demonstrate bactericidal activity at high concentrations.
| Molecular Formula | C46H80N2O13 • H3PO4 |
|---|---|
| Formula Weight | 967.1 |
| Purity | ≥95% |
| Formulation | A crystalline solid |
| Solubility | DMF: 25 mg/ml DMSO: 30 mg/ml PBS (pH 7.2): 10 mg/ml Ethanol: 25 mg/ml |
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.