Tebipenem Pivoxil CAS NO 161715-24-8 Inquire about Tebipenem Pivoxil
Tecoland supplies Tebipenem Pivoxil bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Tebipenem Pivoxil is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Tebipenem Pivoxil 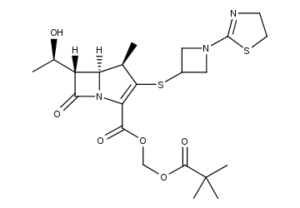
Tebipenem pivoxil is a member of carbapenems and a pivaloyloxymethyl ester.
Tebipenem Pivoxil is an orally available pivaloyloxymethyl ester prodrug of tebipenem, a broad-spectrum 1-beta-methylcarbapenem antibiotic with a 1-(1,3-thiazolin-2-yl) azetidin-3-ylthio group at the C-2 position. After oral administration of tebipenem pivoxil, the ester bond is cleaved, releasing active tebipenem.
Tebipenem pivoxil is an oral carbapenem prodrug that was originated by Wyeth (now Pfizer). It was approved by Pharmaceuticals and Medical Devices Agency of Japan (PMDA) on Apr 22, 2009. It was developed and marketed as Orapenem® by Meiji Seika in Japan. Tebipenem pivoxil is a broad-spectrum orally-administered antibiotic, from the carbapenem subgroup of β-lactam antibiotics. Carbapenems are a class of beta-lactam antibiotics, which act by inhibiting the synthesis of the peptidoglycan layer of bacterial cell walls. It is used to treat otorhinolaryngological infection, otitis media and bacterial pneumonia. Orapenem® is available as granules for oral use, containing 100 mg Tebipenem pivoxil/g granules. According to the weight of children, 4 mg/kg, and twice a day after dinner.
The area under the concentration time curve (AUC) was proportional to the dose in the range of 100–400 mg. The maximum urinary excretion rate was detected at 0–1 or 1–2 h for dose of 100 or 200–400 mg. Cumulative amount of TBPM excreted in urine by 24 h accounted up to 90, 95, and 80% of dose administered for three groups, respectively.