Tasimelteon CAS NO 609799-22-6 Inquire about Tasimelteon
Tecoland supplies Tasimelteon bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Tasimelteon is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Tasimelteon?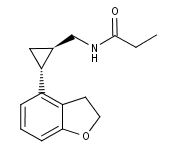
Tasimelteon (trade name Hetlioz) is a drug approved by the FDA solely for the treatment of non-24-hour sleep–wake disorder (often designated as N24HSWD). It is a selective agonist for the melatonin receptors MT1 and MT2 in the suprachiasmatic nucleus of the brain, similar to other members of the melatonin receptor agonist class of which Ramelteon (2005) and agomelatine (2009) were the first approved. In June 2014, the European Medicines Agency accepted an EU filing application for tasimelteon. As a treatment for N24HSWD, as with melatonin or other melatonin derivatives, the patient typically experiences improved sleep quality while taking the drug, but reverts to baseline sleep performance within a month of discontinuation.
Development
Tasimelteon (previously known as BMS-214, 778) was developed for the treatment of insomnia and other sleep disorders. A phase II trial on circadian rhythm sleep disorders was concluded in March 2005. A phase III insomnia trial was conducted in 2006. A second phase III trial on insomnia, this time concerning primary insomnia, was completed in June 2008. In 2010, the FDA granted orphan drug status to tasimelteon, then regarded as an investigational medication, for use in totally blind adults with N24HSWD. This status encourages development of drugs for rare conditions that might otherwise lack sufficient profit motive through mechanisms such as easing the approval process and extending exclusivity periods.
On completion of Phase III trials, interpretations of the clinical trials by the researchers concluded that the drug may have therapeutic potential for transient insomnia in circadian rhythm sleep disorders. Adam Feuerstein writing for The Streetin June 2013 raised significant concerns over the objectivity of the study endpoints, citing relaxed participant screening and moving goalposts. A year-long (2011–2012) study at Harvard tested the use of tasimelteon in blind subjects with non-24-hour sleep–wake disorder. The drug has not been tested in children.
Toxicity
Experiments with rodents revealed fertility impairments, an increase in certain cancers, and serious adverse events during pregnancy at dosages in excess of what is considered the “human dose”
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.