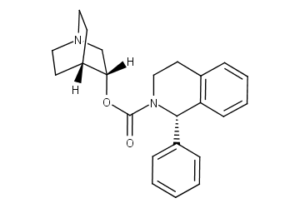
Solifenacin CAS NO 242478-38-2 Inquire about Solifenacin
Tecoland supplies Solifenacin bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Solifenacin is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
Solifenacin is a medicine of the antimuscarinic class and was developed for treating contraction of overactive bladder with associated problems such as increased urination frequency and urge incontinence. Solifenacin is contraindicated for people with urinary retention, gastric retention, uncontrolled or poorly controlled closed-angle glaucoma, severe liver disease and hemodialysis.
Side Effects
The most common side effects of solifenacin are dry mouth, blurred vision, and constipation. As all anticholinergics, solifenacin may rarely cause hyperthermia due to decreased perspiration.
Mechanism of action
Solifenacin is a competitive cholinergic receptor antagonist, selective for the M3 receptor subtype. The binding of acetylcholine to these receptors, particularly M3, plays a critical role in the contraction of smooth muscle. By preventing the binding of acetylcholine to these receptors, solifenacin reduces smooth muscle tone in the bladder, allowing the bladder to retain larger volumes of urine and reducing the number of micturition, urgency and incontinence episodes. Because of a long elimination half life, a once-a-day dose can offer 24-hour control of the urinary bladder smooth muscle tone.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.