Sofosbuvir CAS NO 1190307-88-0 Inquire about Sofosbuvir
Tecoland supplies Sofosbuvir bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Sofosbuvir is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Sofosbuvir?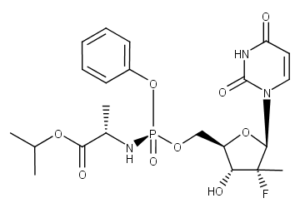
Sofosbuvir (brand names Sovaldi,Hepcinat, Resof, Hepcvir, SoviHep) is a nucleotide analog used in combination with other drugs for the treatment of hepatitis C virus (HCV) infection. It has been marketed since 2013. Compared to previous treatments, sofosbuvir-based regimens provide a higher cure rate, fewer side effects, and a two- to four-fold reduced duration of therapy. Sofosbuvir allows most patients to be treated successfully without the use of peginterferon, an injectable drug with severe side effects that is a key component of older drug combinations for the treatment of HCV.
Sofosbuvir inhibits the RNA polymerase that the hepatitis C virus uses to replicate its RNA. It was discovered at Pharmasset and developed by Gilead Sciences.
Mechanism of action
Sofosbuvir is a prodrug using the ProTide biotechnology strategy. It is metabolized to the active antiviral agent 2′-deoxy-2′-α-fluoro-β-C-methyluridine-5′-triphosphate. The triphosphate serves as a defective substrate for the NS5B protein, which is the viral RNA polymerase, thus acts as an inhibitor of viral RNA synthesis. Prior to the discovery of sofosbuvir, a variety of nucleoside analogs had been examined as antihepatitis C treatments, but these exhibited relatively low potency. This low potency arises in part because the enzymatic addition of the first of the three phosphate groups of the triphosphate is slow. The design of sofosbuvir, based on the protide approach, avoids this slow step by building the first phosphate group into the structure of the drug during synthesis. Additional groups are attached to the phosphorus to temporarily mask the two negative charges of the phosphate group, thereby facilitating entry of the drug into the infected cell. The NS5B protein is a RNA-dependent RNA polymerase critical for the viral reproduction cycle.
Sofosbuvir and other nucleotide inhibitors of the HCV RNA polymerase exhibit a very high barrier to resistance development. This is an important advantage relative to HCV drugs that target other viral enzymes such as the protease, for which rapid resistance development has proved to be an important cause of therapeutic failure.
Adverse effects
As sofosbuvir was combined with other drugs such as ribavirin and interferon in clinical safety trials, only the adverse effects of these combinations have been evaluated. Common side effects are fatigue, headache, nausea, rash, and irritability. Most side effects are significantly more common in interferon-containing regimens as compared to interferon-free ones. For example, fatigue and headache are nearly cut in half, influenza-like symptoms are reduced to 3–6% as compared to 16–18%, and neutropenia is almost absent in interferon-free treatment.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.