Rucaparib CAS NO 283173-50-2 Inquire about Rucaparib
Tecoland supplies Rucaparib bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Rucaparib is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Rucaparib?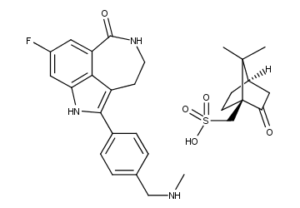
Rucaparib is a PARP inhibitor used as an anti-cancer agent. Rucaparib is a first-in-class pharmaceutical drug targeting the DNA repair enzyme poly-ADP ribose polymerase-1 (PARP-1). It was discovered as part of a collaboration between scientists working at the Northern Institute of Cancer Research and Medical School of Newcastle University and Agouron Pharmaceuticals in San Diego, California. It is being developed by Clovis Oncology. In December 2016, the U.S. FDA granted an accelerated approval for use in cases of pretreated advanced ovarian cancer. In Europe it was designated as an orphan medicinal product on 10 October 2012. On 22 March 2018 the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending the granting of a conditional marketing authorisation, intended for the treatment of relapsed or progressive ovarian cancer. It can be taken orally in tablet form.
Mechanism of Action:
Rucaparib inhibits “the contraction of isolated vascular smooth muscle, including that from the tumours of cancer patients. It also reduces the migration of some cancer and normal cells in culture.” As a PARP inhibitor, rucaparib is expected to be more effective in the 9% of pancreatic cancers with a BRCA mutation (BRCA1 or BRCA2).
Rucaparin Side effects:
● nausea
● fatigue (including weakness)
● vomiting
● anemia
● abdominal pain
● changes in taste
● constipation
● decreased appetite
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.