Rivastigmine Tartrate CAS NO 129101-54-8 Inquire about Rivastigmine Tartrate
Tecoland supplies Rivastigmine Tartrate bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Rivastigmine Tartrate is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Rivastigmine Tartrate?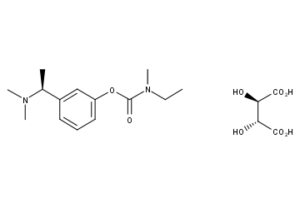
Rivastigmine Tartrate is a reversible cholinesterase inhibitor and is known chemically as (S)-N-Ethyl-N-methyl-3-[1-(dimethylamino)ethyl]-phenyl carbamate hydrogen-(2R, 3R)-tartrate. Rivastigmine tartrate is commonly referred to in the pharmacological literature as SDZ ENA 713 or ENA 713.
Rivastigmine is a parasympathomimetic or cholinergic agent for the treatment of mild to moderate dementia of the Alzheimer’s type. Rivastigmine is a cholinesterase inhibitor that inhibits both butyrylcholinesterase and acetylcholinesterase.
Indication
Rivastigmine Tartrate is used for the treatment of mild to moderate dementia associated with Parkinson’s disease or of the Alzheimer’s type.
What is the mechanism of action?
Rivastigmine is a carbamate derivative that is structurally related to physostigmine, but not to donepezil and tacrine. The precise mechanism of rivastigmine has not been fully determined, but it is suggested that rivastigmine binds reversibly with and inactivates chlolinesterase (eg. acetylcholinesterase, butyrylcholinesterase), preventing the hydrolysis of acetycholine, and thus leading to an increased concentration of acetylcholine at cholinergic synapses. The anticholinesterase activity of rivastigmine is relatively specific for brain acetylcholinesterase and butyrylcholinesterase compared with those in peripheral tissues.
Pharmacodynamics
Rivastigmine is a parasympathomimetic and a reversible cholinesterase inhibitor. An early pathophysiological feature of Alzheimer’s disease that is associated with memory loss and cognitive deficits is a deficiency of acetylcholine as a result of selective loss of cholinergic neurons in the cerebral cortex, nucleus basalis, and hippocampus. Tacrine is postulated to exert its therapeutic effect by enhancing cholinergic function. While the precise mechanism of rivastigmine’s action is unknown, it is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by cholinesterase. If this proposed mechanism is correct, rivastigmine’s effect may lessen as the disease progresses and fewer cholinergic neurons remain functionally intact.
Precautions
Before taking rivastigmine, tell your doctor or pharmacist if you are allergic to it; or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: breathing/lung problems (such as asthma, COPD-chronic obstructive pulmonary disease), stomach/intestinal problems (such as ulcers, bleeding), heart disease (such as sick sinus syndrome, other conduction disorders), fainting, seizures, problems urinating (such as due to enlarged prostate).
How to dose Rivastigmine Tartrate?
The dosage of rivastigmine tartrate shown to be effective in controlled clinical trials in Alzheimer’s Disease is 6-12 mg/day, given as twice-a-day dosing (daily doses of 3 to 6 mg BID). There is evidence from the clinical trials that doses at the higher end of this range may be more beneficial.
The starting dose of rivastigmine tartrate is 1.5 mg twice a day (BID). If this dose is well tolerated, after a minimum of 2 weeks of treatment, the dose may be increased to 3 mg BID. Subsequent increases to 4.5 mg BID and 6 mg BID should be attempted after a minimum of 2 weeks at the previous dose. If adverse effects (e.g., nausea, vomiting, abdominal pain, loss of appetite) cause intolerance during treatment, the patient should be instructed to discontinue treatment for several doses and then restart at the same or next lower dose level. If treatment is interrupted for longer than several days, treatment should be reinitiated with the lowest daily dose. The maximum dose is 6 mg BID (12 mg/day).
What are the side effects of Rivastigmine Tartrate?
Get emergency medical help if you have any of these signs of an allergic reaction: hives; difficulty breathing; swelling of your face, lips, tongue, or throat.
Stop using rivastigmine and call your doctor at once if you have any of these serious side effects:
- stomach pain, nausea and vomiting, loss of appetite;
- black, bloody, or tarry stools;
- coughing up blood or vomit that looks like blood or coffee grounds;
- feeling light-headed, fainting;
- chest pain;
- confusion, agitation, extreme fear.
Storage
Store rivastigmine tartrate at room temperature away from air and heat.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.