Pramipexole Di-HCl CAS NO 104632-25-9 Inquire about Pramipexole Di-HCl
Tecoland supplies Pramipexole Di-HCl bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Pramipexole Di-HCl is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Pramipexole Di-HCl 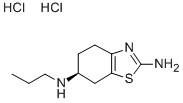
Pramipexole Dihydrochloride is the hydrochloride salt of pramipexole, a benzothiazole derivative. As a nonergot dopamine agonist, pramipexole binds to D2 and D3 dopamine receptors in the striatum and substantia nigra of the brain. Compared to other dopamine agonists, the use of this agent may be associated with fewer dyskinetic side effects in treated subjects.
Pramipexole is used alone or with other medications to treat Parkinson’s disease. It can improve your ability to move and decrease shakiness (tremor), stiffness, slowed movement, and unsteadiness. It may also decrease the number of episodes of not being able to move (“on-off syndrome”).This medication is also used to treat a certain medical condition (restless legs syndrome – RLS) that causes an unusual urge to move the legs. Symptoms usually occur at night along with uncomfortable/unpleasant feelings in the legs. This medication can decrease these symptoms and thereby improve sleep. Pramipexole is a dopamine agonist that works by helping to restore the balance of a certain natural substance (dopamine) in the brain.
Indications and Usage for Pramipexole
Parkinson’s Disease
Pramipexole dihydrochloride tablets are indicated for the treatment of Parkinson’s disease.
Restless Legs Syndrome
Pramipexole dihydrochloride tablets are indicated for the treatment of moderate-to-severe primary Restless Legs Syndrome (RLS).
Drug Interactions
Dopamine Antagonists
Since Pramipexole is a dopamine agonist, it is possible that dopamine antagonists, such as the neuroleptics (phenothiazines, butyrophenones, thioxanthenes) or metoclopramide, may diminish the effectiveness of Pramipexole dihydrochloride tablets.
Overdosage
There is no clinical experience with significant overdosage. One patient took 11 mg/day of Pramipexole for 2 days in a clinical trial for an investigational use. Blood pressure remained stable although pulse rate increased to between 100 and 120 beats/minute. No other adverse reactions were reported related to the increased dose.
There is no known antidote for overdosage of a dopamine agonist. If signs of central nervous system stimulation are present, a phenothiazine or other butyrophenone neuroleptic agent may be indicated; the efficacy of such drugs in reversing the effects of overdosage has not been assessed. Management of overdose may require general supportive measures along with gastric lavage, intravenous fluids, and electrocardiogram monitoring.
Adverse Reactions
- Falling Asleep During Activities of Daily Living and Somnolence.
- Symptomatic Orthostatic Hypotension.
- Impulse Control/Compulsive Behaviors.
- Hallucinations and Psychotic-like Behavior.
- Dyskinesia.
- Postural Deformity.
- Rhabdomyolysis.
- Retinal Pathology.
- Events Reported with Dopaminergic Therapy.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.