Pirfenidone CAS NO 53179-13-8 Inquire about Pirfenidone
Tecoland supplies Pirfenidone bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Pirfenidone is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Pirfenidone?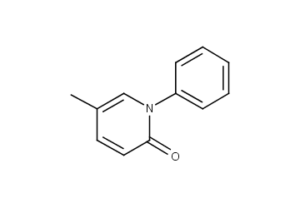
Pirfenidone is a medication used for the treatment of idiopathic pulmonary fibrosis. It works by reducing lung fibrosis through downregulation of the production of growth factorsand procollagens I and II. In October 2010, the Indian Company Cipla launched it as Pirfenex. In 2011, it was approved for use in Europe for idiopathic pulmonary fibrosis under the trade name Esbriet; it was approved in Canada in 2012 under the trade name Esbriet; and was approved in the United States in October 2014 under the same name. In September 2011, the Chinese State Food and Drug Administration provided GNI Group Ltd with new drug approval of pirfenidone in China,and later manufacture approval in 2013 under the trade name of Etuary.In 2014 it was approved in Mexico under the name KitosCell LP, indicated for pulmonary fibrosis and liver fibrosis. There is also a topical form created for the treatment of abnormal wound healing processes.
Mechanism of Action:
Pirfenidone has well-established antifibrotic and anti-inflammatory properties in various in vitro systems and animal models of fibrosis. A number of cell-based studies have shown that pirfenidone reduces fibroblast proliferation, inhibits transforming growth factor beta stimulated collagen production and reduces the production of fibrogenic mediators such as transforming growth factor beta. Pirfenidone has also been shown to reduce production of inflammatory mediators such as tumor necrosis factor alpha and IL-1β in both cultured cells and isolated human peripheral blood mononuclear cells. These activities are consistent with the broader antifibrotic and anti-inflammatory activities observed in animal models of fibrosis.
Pharmacokinetics:
Pirfenidone is administered orally. Though the presence of food significantly reduces the extent of absorption, the drug is to be taken after food, to reduce the nausea and dizziness associated with the drug. The drug is around 60% bound to plasma proteins, especially to albumin. Up to 50% of the drug is metabolized by hepatic CYP1A2 enzyme system to yield 5-carboxypirfenidone, the inactive metabolite. Almost 80% of the administered dose is excreted in the urine within 24 hours of intake.
Medical use:
In Europe, pirfenidone is indicated for the treatment of mild-to-moderate idiopathic pulmonary fibrosis. It was approved by the European Medicines Agency in 2011. In October 2008, it was approved for use in Japan, in India in 2010, and in China in 2011 (commercial launch in 2014). In October 2014, it was approved for sale in the United States. In Mexico it has been approved on a ge] form for the treatment of scars and fibrotic tissue and has proven to be effective in the treatment of skin ulcers
Pirfenidone Side effects:
● Gastrointestinal
● Skin-rash, pruritus and dry skin
● Hepatic dysfunction
● Dizziness and fatigue
● Weight loss
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.