Ozanimod CAS NO 1306760-87-1 Inquire about Ozanimod
Tecoland supplies Ozanimod bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Ozanimod is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Ozanimod?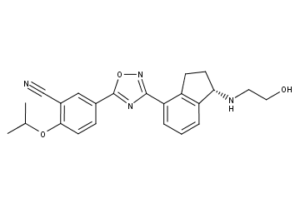
Ozanimod (RPC-1063) is an investigational immunomodulatory drug currently in phase III clinical trials for the therapy of relapsing multiple sclerosis (RMS) and ulcerative colitis (UC). It acts as a sphingosine-1-phosphate (S1P) receptor agonist, sequestering lymphocytes to peripheral lymphoid organs and away from their sites of chronic inflammation.
Pharmacodynamics:
Ozanimod is an agonist of the S1P1 and S1P5 receptors. It demonstrates this effect in a dose-dependent manner, with 10-fold potency to three comparators.This is an improvement of selectivity over its predecessor, fingolimod, which is non-specific to all 5 isotypes. The agonism of S1P directly causes its internalization and degradation through the ubiquitin-proteosome pathway.The loss of S1P leads to a decrease in the total lymphocyte count in circulation, specifically CD4+CCR7+ and CD8+ CCR7+ T cells.
Pharmacokinetics:
Ozanimod has a high oral bioavailability, a circulating half-life of about 19 hours, and reaches highest blood plasma concentrations after about 6 hours.Ozanimod is dehydrogenated by two CYP enzymes into two active metabolites, all with similar pharmacokinetics. The decrease in lymphocyte count lasts for approximately 14 days after treatment discontinuation. Unlike fingolimod, it does not require phosphorylation for activation, nor does it demonstrate cardiac abnormalities or hepatotoxicity.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.