Niraparib CAS NO 1038915-60-4 Inquire about Niraparib
Tecoland supplies Niraparib bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Niraparib is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Niraparib?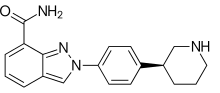
Niraparib is an orally active PARP inhibitor developed by Tesaro to treat ovarian cancer. FDA approval on March 2017.
Mechanism of Action:
Niraparib is an inhibitor of poly (ADP-ribose) polymerase (PARP) enzymes, PARP-1 and PARP-2, which play a role in DNA repair. In vitro studies have shown that niraparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complexes resulting in DNA damage, apoptosis and cell death. Increased niraparib-induced cytotoxicity was observed in tumor cell lines with or without deficiencies in BRCA1/2. Niraparib decreased tumor growth in mouse xenograft models of human cancer cell lines with deficiencies in BRCA1/2 and in human patient-derived xenograft tumor models with homologous recombination deficiency that had either mutated or wild type BRCA1/2.
Pharmacodynamics:
Cardiovascular Effects: Niraparib has the potential to cause effects on pulse rate and blood pressure in patients receiving the recommended dose, which may be related to pharmacological inhibition of the dopamine transporter (DAT), norepinephrine transporter (NET) and serotonin transporter (SERT). In the NOVA study, mean pulse rate and blood pressure increased over baseline in the niraparib arm relative to the placebo arm at all on-study assessments.
Absorption:
The absolute bioavailability of niraparib is approximately 73%. Following oral administration of niraparib, peak plasma concentration, Cmax, is reached within 3 hours. Concomitant administration of a high fat meal (800-1,000 calories with approximately 50% of total caloric content of the meal from fat) did not significantly affect the pharmacokinetics of niraparib.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.