Neratinib CAS NO 698387-09-6 Inquire about Neratinib
Tecoland supplies Neratinib bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Neratinib is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Neratinib?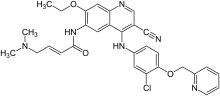
Neratinib was approved in July 2017 for use as an extended adjuvant therapy in Human Epidermal Growth Factor Receptor 2 (HER2) positive breast cancer. Approval was granted to Puma Biotechnology Inc. for the tradename Nerlynx. Neratinib is currently under investigation for use in many other forms of cancer.
Mechanism of Action:
Neratinib binds to and irreversibly inhibits EGFR, HER2, and HER4. This prevents autophosphorylation of tyrosine residues on the receptor and reduces oncogenic signalling through the mitogen-activated protein kinase and Akt pathways.
Medical use:
Neratinib is used as an adjuvant therapy in people with early stage breast cancer in which HER2 is overexpressed, after the person receives treatment with trastuzumab.
Neratinib Side effects:
Neratinib can cause life-threatening diarrhea in some people and mild to moderate diarrhea in almost everyone; people who take it are also at risk for complications of diarrhea like dehydration and electrolyte imbalance.Similarly there is a risk of severe liver damage and many patients have some level of it; symptoms of liver damage include fatigue, nausea, vomiting, right upper quadrant pain or tenderness, fever, rash, and high levels of eosinophils.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.