Mycophenolate Sodium CAS NO 37415-62-6 Inquire about Mycophenolate Sodium
Tecoland supplies Mycophenolate Sodium bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Mycophenolate Sodium is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Mycophenolate Sodium 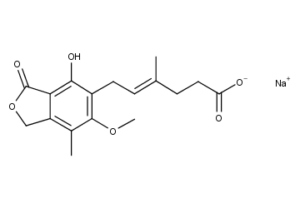
Mycophenolate Sodium is the sodium salt form of mycophenolic acid (MPA), with immunosuppressing activity. In vivo, the active molecule MPA reversibly inhibits inosine monophosphate dehydrogenase (IMPDH) which is needed for guanine monophosphate synthesis and stops the proliferation of B and T lymphocytes. Relative to other cell types, lypmocytes are highly dependent on salvage and de novo synthesis of guanine nucleotides, thus making these cells prone to MPA cytotoxicity.
Mycophenolate sodium is an organic sodium salt that is the sodium salt of mycophenolic acid. An immunosuppressant, it is widely used to prevent tissue rejection following organ transplants and for the treatment of certain autoimmune diseases. It has a role as an EC 1.1.1.205 (IMP dehydrogenase) inhibitor and an immunosuppressive agent. It contains a mycophenolate.
Compound derived from Penicillium stoloniferum and related species. It blocks de novo biosynthesis of purine nucleotides by inhibition of the enzyme inosine monophosphate dehydrogenase (IMP DEHYDROGENASE). Mycophenolic acid exerts selective effects on the immune system in which it prevents the proliferation of T-CELLS, LYMPHOCYTES, and the formation of antibodies from B-CELLS. It may also inhibit recruitment of LEUKOCYTES to sites of INFLAMMATION.
Common Side Effects
- Black, tarry stools.
- bladder pain.
- blood in the urine or stools.
- burning, crawling, itching, numbness, prickling, “pins and needles”, or tingling feelings.
- chest pain or tightness.
- cough or hoarseness.
- decreased urine.
- difficult or labored breathing.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.