Lurasidone HCl CAS NO 367514-88-3 Inquire about Lurasidone HCl
Tecoland supplies Lurasidone HCl bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Lurasidone HCl is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Lurasidone HCl?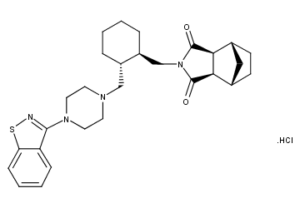
Lurasidone HCl is an atypical antipsychotic agent indicated for the treatment of patients with schizophrenia.
Lurasidone HCl is a psychotropic agent belonging to the chemical class of benzoisothiazol derivatives. Its chemical name is (3aR,4S,7R,7aS)-2-{(1R,2R)-2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-ylmethyl] cyclohexylmethyl}hexahydro-4,7-methano-2H-isoindole-1,3-dione hydrochloride. Its molecular formula is C28H36N4O2S·HCl and its molecular weight is 529.14.
Lurasidone hydrochloride is a white to off-white powder. It is very slightly soluble in water, practically insoluble or insoluble in 0.1 N HCl, slightly soluble in ethanol, sparingly soluble in methanol, practically insoluble or insoluble in toluene and very slightly soluble in acetone.
Lurasidone HCl Drug reactions
Potential for Other Drugs to Affect lurasidone HCl
Lurasidone HCl is not a substrate of CYP1A1, CYP1A2, CYP2A6, CYP4A11, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP2E1 enzymes. This suggests that an interaction of lurasidone HCl with drugs that are inhibitors or inducers of these enzymes is unlikely.
Lurasidone HCl is predominantly metabolized by CYP3A4; interaction of lurasidone HCl with strong and moderate inhibitors or inducers of this enzyme has been observed. Lurasidone HCl should not be used in combination with strong inhibitors or inducers of this enzyme.
Potential for lurasidone HCl to Affect Other Drugs
Digoxin (P-gp substrate): Coadministration of lurasidone HCl (120 mg/day) at steady state with a single dose of digoxin (0.25 mg) increased Cmax and AUC(0-24) for digoxin by approximately 9% and 13%, respectively relative to digoxin alone. Digoxin dose adjustment is not required when coadministered with lurasidone HCl.
Midazolam (CYP3A4 substrate): Coadministration of lurasidone HCl (120 mg/day) at steady state with a single dose of 5 mg midazolam increased midazolam Cmax and AUC(0-24) by approximately 21% and 44%, respectively relative to midazolam alone. Midazolam dose adjustment is not required when coadministered with lurasidone HCl.
Oral Contraceptive (estrogen/progesterone): Coadministration of lurasidone HCl (40 mg/day) at steady state with an oral contraceptive (OC) containing ethinyl estradiol and norelgestimate resulted in equivalent AUC(0-24) and Cmax of ethinyl estradiol and norelgestromin relative to OC administration alone. Also, sex hormone binding globulin levels were not meaningfully affected by coadministration of lurasidone HCl and OC. Dose adjustment of OC dose is not required when coadministered with lurasidone HCl.
Lurasidone HCl precautions
- Cerebrovascular Adverse Reactions: An increased incidence of cerebrovascular adverse events (e.g., stroke, transient ischemic attack) has been seen in elderly patients with dementia-related psychoses treated with atypical antipsychotic drugs.
- Neuroleptic Malignant Syndrome: Manage with immediate discontinuation and close monitoring.
- Tardive Dyskinesia: Discontinue if clinically appropriate.
- Metabolic Changes: Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and weight gain.
- Hyperglycemia and Diabetes Mellitus: Monitor patients for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Monitor glucose regularly in patients with diabetes or at risk for diabetes.
- Dyslipidemia: Undesirable alterations have been observed in patients treated with atypical antipsychotics.
- Weight Gain: Gain in body weight has been observed, clinical monitoring of weight is recommended.
- Hyperprolactinemia: Prolactin elevations may occur.
- Leukopenia, Neutropenia, and Agranulocytosis have been reported with antipsychotics. Patients with a pre-existing low white blood cell count (WBC) or a history of leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and lurasidone HCl should be discontinued at the first sign of a decline in WBC in the absence of other causative factors.
- Orthostatic Hypotension and Syncope: Dizziness, tachycardia or bradycardia, and syncope may occur, especially early in treatment. Use with caution in patients with known cardiovascular or cerebrovascular disease, and in antipsychotic-naïve patients.
- Seizures: Use cautiously in patients with a history of seizures or with conditions that lower the seizure threshold.
- Potential for Cognitive and Motor Impairment: Use caution when operating machinery.
- Suicide: The possibility of a suicide attempt is inherent in schizophrenia. Closely supervise high-risk patients.
Lurasidone HCl side effects
Commonly observed adverse reactions (incidence ?5% and at least twice the rate for placebo) included somnolence, akathisia, nausea, parkinsonism and agitation.
Lurasidone HCl Storage
Store lurasidone HCl at 25°C (77°F); excursions permitted to 15° – 30°C (59° – 86°F) [See USP Controlled Room Temperature].
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.