Lenvatinib CAS NO 417716-92-8 Inquire about Lenvatinib
Tecoland supplies Lenvatinib bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Lenvatinib is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Lenatinib 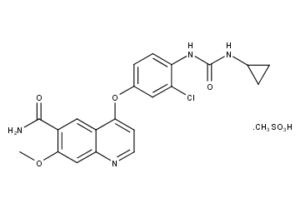
Lenvatinib is a kinase inhibitor used to treat certain types of cancer. Kinase inhibitors are enzyme inhibitors that blocks the action of one or more protein kinases.
Lenvatinib is used to treat thyroid cancer. It is usually given after radioactive iodine has been tried without success.
Lenvatinib is used together with everolimus (Afinitor) to treat advanced kidney cancer when other medicines have not been effective.
Lenvatinib is used together with pembrolizumab (Keytruda) to treat a certain type of endometrial cancer (a type of uterine cancer) that has progressed and cannot be removed with surgery or radiation.
Lenvatinib is also used to treat liver cancer that cannot be removed with surgery.
Warnings
Some people taking lenvatinib have developed a perforation (a hole or tear) or a fistula (an abnormal passageway) within the stomach or intestines. Get emergency medical help if you have severe stomach pain, or if you feel like you are choking and gagging when you eat or drink.
Call your doctor at once if you have signs of serious side effects, including: severe chest pain, shortness of breath, swelling in your ankles, numbness or weakness, confusion, severe headache, problems with speech or vision, seizure (convulsions), unusual bleeding, coughing up blood, dark urine, clay-colored stools, or jaundice (yellowing of the skin or eyes).
Before taking this medicine
To make sure lenvatinib is safe for you, tell your doctor if you have ever had:
- long QT syndrome (in you or a family member);
- heart disease, high blood pressure;
- a heart attack, heart failure, stroke, or blood clot;
- headaches or vision problems;
- bleeding problems;
- a perforation (a hole or tear) in your stomach or intestines;
- a seizure disorder;
- a recent surgery or if you plan to have surgery or a dental procedure;
- kidney disease; or
- liver disease.
Lenvatinib may cause jaw bone problems (osteonecrosis). The risk is highest in people with cancer, blood cell disorders, pre-existing dental problems, or people treated with steroids, chemotherapy, or radiation. Ask your doctor about your own risk.
Lenvatinib may harm an unborn baby. You may need to have a negative pregnancy test before starting this treatment. Use effective birth control to prevent pregnancy while you are using this medicine and for at least 30 days after your last dose.
Pregnancy may be less likely to occur while the mother or the father is using this medicine. Both men and women should still use birth control to prevent pregnancy because the medicine can harm an unborn baby.
Lenvatinib may affect fertility in men or women. Pregnancy could be harder to achieve while either parent is using this medicine.
Do not breastfeed while using this medicine, and for at least 1 week after your last dose.
Lenvatinib Side Effects
Get emergency medical help if you have signs of an allergic reaction to lenvatinib: hives; difficult breathing; swelling of your face, lips, tongue, or throat.
Some people taking lenvatinib have developed a perforation (a hole or tear) or a fistula (an abnormal passageway) within the stomach or intestines. Call your doctor if you have severe stomach pain, or if you feel like you are choking and gagging when you eat or drink.
Also call your doctor at once if you have:
- severe diarrhea;
- headache, confusion, change in mental status, vision loss, seizure (convulsions);
- pain or burning when you urinate, urinating less;
- increased protein in your urine (proteinuria);
- irregular heartbeats;
- unusual bleeding (nosebleeds, heavy menstrual bleeding), or any other bleeding that will not stop;
- severe headache, blurred vision, pounding in your neck or ears;
- jaw pain or numbness, red or swollen gums, loose teeth, or slow healing after dental work;
- signs of stomach bleeding – bloody or tarry stools, coughing up blood or vomit that looks like coffee grounds;
- heart problems – chest pain, pain in your jaw or shoulder, swelling, rapid weight gain, feeling short of breath;
- signs of a blood clot – sudden numbness or weakness, problems with vision or speech;
- liver problems – dark urine, clay-colored stools, jaundice (yellowing of the skin or eyes); or
- low calcium level – muscle spasms or contractions, numbness or tingly feeling (around your mouth, or in your fingers and toes).
Your cancer treatments may be delayed or permanently discontinued if you have certain side effects.
Common lenvatinib side effects may include:
- bleeding;
- stomach pain, nausea, vomiting, diarrhea;
- loss of appetite, weight loss;
- abnormal thyroid function tests;
- muscle or joint pain;
- pain and burning when you urinate;
- swelling in your arms and legs;
- mouth sores;
- rash;
- redness, itching, or peeling skin on your hands or feet;
- headache, tiredness; or
- cough, trouble breathing, hoarse voice.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Drug Interactions
Lenvatinib can cause a serious heart problem. Your risk may be higher if you also use certain other medicines for infections, asthma, heart problems, high blood pressure, depression, mental illness, cancer, malaria, or HIV.
Tell your doctor about all your other medicines, especially an osteoporosis medicine.
Other drugs may interact with lenvatinib, including prescription and over-the-counter medicines, vitamins, and herbal products. Tell your doctor about all other medicines you use.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.