Ledipasvir CAS NO 1256388-51-8 Inquire about Ledipasvir
Tecoland supplies Ledipasvir bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Ledipasvir is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Ledipasvir?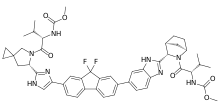
Ledipasvir is a drug for the treatment of hepatitis C that was developed by Gilead Sciences. After completing Phase III clinical trials, on February 10, 2014 Gilead filed for U.S. approval of a ledipasvir/sofosbuvir fixed-dose combination tablet for genotype 1 hepatitis C. The ledipasvir/sofosbuvir combination is a direct-acting antiviral agent that interferes with HCV replication and can be used to treat patients with genotypes 1a or 1b without PEG-interferon or ribavirin. Ledipasvir is an inhibitor of NS5A, a hepatitis C virus protein. On 10 October 2014 the FDA approved the combination product ledipasvir/sofosbuvir called Harvoni.
Mechanism of Action:
Ledipasvir inhibits an important viral phosphoprotein, NS5A, which is involved in viral replication, assembly, and secretion. Sofosbuvir, on the other hand, is metabolized to a uridine triphosphate mimic, which acts as a RNA chain terminator when incorporated into RNA by NS5B polymerase.
Ledipasvir Side effects:
Fatigue, headache, nausea, diarrhea, insomnia, and weakness.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.