Latanoprost CAS NO 130209-82-4 Inquire about Latanoprost
Tecoland supplies Latanoprost bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Latanoprost is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Latanoprost?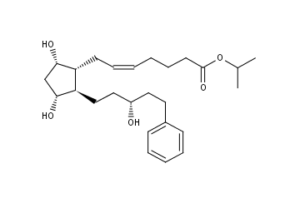
Latanoprost (pronounced la-TAN-oh-prost) ophthalmic solution is a medication administered into the eyes to control the progression of glaucoma or ocular hypertension by reducing intraocular pressure. It is a prostaglandin analogue (more specifically an analogue of prostaglandin F2?) that lowers the pressure by increasing the outflow of aqueous fluid from the eyes through the uvealsclearal tract. Latanoprost is an isopropyl ester prodrug, meaning it is inactive until it is hydrolyzed by esterases in the cornea to the biologically active acid. It is also known by the brand name of Xalatan manufactured by Pfizer. Annual sales are approximately $1.6 billion. The patent for latanoprost expired in March 2011, and at least one generic version (manufactured by Mylan Inc.) is now widely available in the U.S.
Latanoprost was invented by Johan W. Stjernschantz and Bahram Resul, employees of the Pharmacia Corporation of Uppsala, Sweden. It is on the World Health Organization’s List of Essential Medicines, a list of the most important medication needed in a basic health system.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.