Isepamicin Sulfate CAS NO 67814-76-0 Inquire about Isepamicin Sulfate
Tecoland supplies Isepamicin Sulfate bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Isepamicin Sulfate is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Isepamicin Sulfate 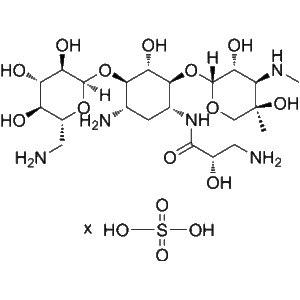
Isepamicin Sulphate (Isepamicine, Isepamycin, sch21420) is an aminoglycoside antibiotic, which inhibits bacterial protein synthesis by targeting the bacterial 30S ribosomal subunit.
Isepamicin is an aminoglycoside antibacterial with properties similar to those of amikacin, but with better activity against strains producing type I 6′-acetyltransferase. The antibacterial spectrum includes Enterobacteriaceae and staphylococci. Anaerobes, Neisseriaceae and streptococci are resistant. The lower and upper break-points are 8 and 16 mg/L. Like other aminoglycosides, isepamicin exhibits a strong concentration-dependent bactericidal effect, a long post-antibiotic effect (several hours) and induces adaptive resistance. Isepamicin is administered intravenously or intramuscularly at a dosage of 15 mg/kg once daily or 7.5 mg/kg twice daily. Isepamicin is not bound to plasma proteins, and it distributes in extracellular fluids and into some cells (outer hair cells, kidney cortex) by active transport. Isepamicin has been developed and approved for clinical use in the 1990s.
Use Guides
In Vivo Use Guide
Parenteral Susceptible infections Adult: Up to 15 mg/kg daily by IM injection or IV infusion in 2 divided doses. Adjust dose based on serum isepamicin conc monitoring. Total dose should not exceed 1.5 g/day. Max Dosage: 1.5 g daily.
Route of Administration: Parenteral.
In Vitro Use Guide
The 90% minimum inhibitory concentration (MIC90) ranged from 1.1 to 8.5 mg/L for members of the Enterobacteriaceae. Pseudomonas aeruginosa and Acinetobacter spp. have MIC90 values of 7.8 and 7.2 mg/L, respectively.
Side Effects
Ototoxicity; nephrotoxicity; electrolyte disturbances; respiratory depression; muscular paralysis; cross-sensitivity with other aminoglycosides.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.