Iomeprol CAS NO 78649-41-9 Inquire about Iomeprol
Tecoland supplies Iomeprol bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Iomeprol is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Iomeprol 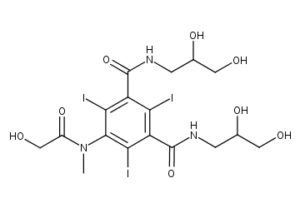
Iomeprol is a pharmaceutical drug used as a radiocontrast agent in X-ray imaging. It is classified as a water-soluble, nephrotrophic, low osmolar X-ray contrast medium. Low osmolar non-ionic agents are better tolerated and less likely to cause side effects than the high osmolar ionic agents. The substance is not metabolized in the human body but excreted in unchanged form. It is decomposed slowly and can therefore accumulate in the environment.
Iomeprol is a nonionic, monomeric iodinated contrast medium. Unlike the older ionic agents, iomeprol has low chemotoxicity, osmolality and viscosity and high water solubility. Compared with other nonionic contrast media, the osmolality and viscosity are lower and the water solubility is reported to be higher with iomeprol.
Iomeprol (containing 150 to 400 mg/ml of iodine) was well tolerated in clinical trials. Most adverse events were transient and of mild to moderate intensity and were similar to those observed with other contrast media. The overall incidence of adverse events ranged from 3 to 49.7% and mainly included localised pain (< or =6%) and heat sensations (8 to 45%), taste disturbances (3 to 27%) and various pseudoallergic reactions (< or =20% for each type of event). The incidence of heat or pain and taste disturbances with iomeprol was similar to that observed with iopromide and iopamidol. Pain (but not heat sensations) was reported significantly less frequently and taste disturbances reported significantly more frequently with iomeprol than with iohexol in a comparative trial. Pseudoallergic reactions (such as nausea, vomiting, skin reactions, dizziness, headache) were significantly less common with iomeprol than with ioxaglate and occurred at a similar frequency to that with iopromide and iopamidol. Cardiovascular events were rarely observed with iomeprol.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.