Iloperidone CAS NO 133454-47-4 Inquire about Iloperidone
Tecoland supplies Iloperidone bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Iloperidone is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Iloperidone?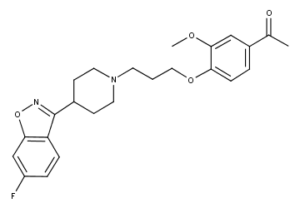
Iloperidone is a psychotropic agent belonging to the chemical class of piperidinyl-benzisoxazole derivatives. Its chemical name is 4’-[3-[4-(6-Fluoro-1, 2-benzisoxazol-3-yl)piperidino]propoxy]-3’-methoxyacetophenone. Its molecular formula is C24H27FN2O4 and its molecular weight is 426.48.
Iloperidone is a white to off-white finely crystalline powder. It is practically insoluble in water, very slightly soluble in 0.1 N HCl and freely soluble in chloroform, ethanol, methanol, and acetonitrile.
Iloperidone is an atypical antipsychotic agent indicated for the treatment of schizophrenia in adults. (1) Efficacy was established in two short-term (4- and 6-week) placebo- and active-controlled studies of adult patients with schizophrenia. In choosing among treatments, prescribers should consider the ability of Iloperidone to prolong the QT interval and the use of other drugs first. Prescribers should also consider the need to titrate Iloperidone slowly to avoid orthostatic hypotension, which may lead to delayed effectiveness compared to some other drugs that do not require similar titration.
Iloperidone Drug Interaction
Potential for Other Drugs to Affect Iloperidone
Iloperidone is not a substrate for CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP2E1 enzymes. This suggests that an interaction of iloperidone with inhibitors or inducers of these enzymes, or other factors, like smoking, is unlikely.
Both CYP3A4 and CYP2D6 are responsible for iloperidone metabolism. Inhibitors of CYP3A4 (e.g., ketoconazole) or CYP2D6 (e.g., fluoxetine, paroxetine) can inhibit iloperidone elimination and cause increased blood levels.
Potential for Iloperidone to Affect Other Drugs
In vitro studies in human liver microsomes showed that iloperidone does not substantially inhibit the metabolism of drugs metabolized by the following cytochrome P450 isozymes: CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, or CYP2E1. Based on in vitro studies, iloperidone is a time-dependent inhibitor of CYP3A at therapeutic exposure levels. Co-administration of iloperidone may lead to an increase in plasma levels of drugs that are predominantly eliminated by CYP3A4. Furthermore, in vitro studies in human liver microsomes showed that iloperidone does not have enzyme inducing properties, specifically for the following cytochrome P450 isozymes: CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP3A4 and CYP3A5.
Drugs that Prolong the QT Interval
Iloperidone should not be used with any other drugs that prolong the QT interval
Precautions Before Using Iloperidone
- Elderly patients with dementia-related psychosis who are treated with atypical antipsychotic drugs are at an increased risk of death and cerebrovascular-related adverse events, including stroke.
- QT prolongation: Prolongs QT interval and may be associated with arrhythmia and sudden death-consider using other antipsychotics first. Avoid use of iloperidone in combination with other drugs that are known to prolong QTc; use caution and consider dose modification when prescribing iloperidone with other drugs that inhibit iloperidone metabolism. Monitor serum potassium and magnesium in patients at risk for electrolyte disturbances.
- Neuroleptic Malignant Syndrome: Manage with immediate discontinuation of drug and close monitoring.
- Tardive dyskinesia: Discontinue if clinically appropriate.
- Metabolic Changes: Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/ cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and weight gain.
- Hyperglycemia and diabetes mellitus: Monitor patients for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Monitor glucose regularly in patients at risk for diabetes.
- Dyslipidemia: Undesirable alterations have been observed in patients treated with atypical antipsychotics.
- Weight Gain: Weight gain has been reported. Monitor weight.
- Seizures: Use cautiously in patients with a history of seizures or with conditions that lower seizure threshold.
- Orthostatic hypotension: Dizziness, tachycardia, and syncope can occur with standing.
- Leukopenia, Neutropenia, and Agranulocytosis have been reported with antipsychotics. Patients with a pre-existing low white blood cell count (WBC) or a history of leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue iloperidone at the first sign of a decline in WBC in the absence of other causative factors.
- Suicide: Close supervision of high risk patients.
- Priapism: Cases have been reported in association with iloperidone treatment.
- Potential for cognitive and motor impairment: Use caution when operating machinery.
Iloperidone side effects
Commonly observed adverse reactions (incidence ?5% and two-fold greater than placebo) were: dizziness, dry mouth, fatigue, nasal congestion, orthostatic hypotension, somnolence, tachycardia, and weight increased.
Iloperidone Overdosage
There is no specific antidote for iloperidone. Therefore appropriate supportive measures should be instituted. In case of acute overdose, the physician should establish and maintain an airway and ensure adequate oxygenation and ventilation. Gastric lavage (after intubation, if patient is unconscious) and administration of activated charcoal together with a laxative should be considered. The possibility of obtundation, seizures or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis. Cardiovascular monitoring should commence immediately and should include continuous ECG monitoring to detect possible arrhythmias. If antiarrhythmic therapy is administered, disopyramide, procainamide and quinidine should not be used, as they have the potential for QT-prolonging effects that might be additive to those of iloperidone. Similarly, it is reasonable to expect that the alpha-blocking properties of bretylium might be additive to those of iloperidone, resulting in problematic hypotension. Hypotension and circulatory collapse should be treated with appropriate measures such as intravenous fluids or sympathomimetic agents (epinephrine and dopamine should not be used, since beta stimulation may worsen hypotension in the setting of FANAPT-induced alpha blockade). In cases of severe extrapyramidal symptoms, anticholinergic medication should be administered. Close medical supervision should continue until the patient recovers.
Iloperidone Storage
Store iloperidone tablets at controlled room temperature, 25°C (77°F); excursions permitted to 15° – 30 °C (59° – 86°F) [See USP Controlled Room Temperature]. Protect iloperidone from exposure to light and moisture.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.