Glycopyrronium bromide CAS NO 51186-83-5 Inquire about Glycopyrronium bromide
Tecoland supplies Glycopyrronium bromide bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Glycopyrronium bromide is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Glycopyrronium bromide 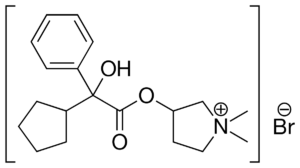
Glycopyrrolate is a synthetic quaternary ammonium that is an anticholinergic agent with antispasmodic activity. Glycopyrrolate competitively binds to peripheral muscarinic receptors in the autonomic effector cells of, and inhibits cholinergic transmission in smooth muscle, cardiac muscle, the sinoatrial (SA) node, the atrioventricular (AV) node, exocrine glands and in the autonomic ganglia. Blockage of cholinergic transmission, in smooth muscle cells located in the gastrointestinal tract and the bladder, causes smooth muscle relaxation and prevents the occurrence of painful spasms. In addition, glycopyrrolate inhibits the release of gastric, pharyngeal, tracheal, and bronchial secretions.
Glycopyrronium bromide is a medication of the muscarinic anticholinergic group. It does not cross the blood–brain barrier and consequently has few to no central effects. It is given by mouth, via intravenous injection, on the skin, and via inhalation. It is a synthetic quaternary ammonium compound. The cation, which is the active moiety, is called glycopyrronium (INN) or glycopyrrolate (USAN).
Glycopyrrolate is an anticholinergic agent used to treat gastrointestinal conditions associated with intestinal spasm and to decrease secretions during anesthesia. Glycopyrrolate has not been implicated in causing liver enzyme elevations or clinically apparent acute liver injury.
Glycopyrronium Bromide Side Effects
The most common side effects include irritability, flushing, blocked nose, reduced secretions in the airways, dry mouth, constipation, diarrhea, vomiting and inability to completely empty the bladder (urinary retention).
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.