Gadoterate Meglumine CAS NO 92943-93-6 Inquire about Gadoterate Meglumine
Tecoland supplies Gadoterate Meglumine bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Gadoterate Meglumine is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Gadoterate Meglumine 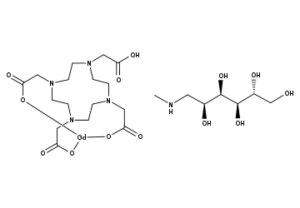
Gadoterate meglumine is a contrast agent that has magnetic properties. It is used in combination with magnetic resonance imaging (MRI) to allow blood vessels, organs, and other non-bony tissues to be seen more clearly on the MRI.
Gadoterate meglumine is used to help diagnose certain disorders of the brain and spine (central nervous system).
Warnings
Gadoterate meglumine can cause a life-threatening condition in people with advanced kidney disease. You should not receive this medication if you have kidney disease or if you are on dialysis.
Before taking this medicine
Gadoterate meglumine can cause a life-threatening condition in people with advanced kidney disease. You should not receive gadoterate meglumine if you have kidney disease or if you are on dialysis.
Gadoterate meglumine can stay in your body for months or years after you receive this medicine. It is not known whether this could cause any health problems in people whose kidneys work properly. Tell your doctor if you have had repeated scans with a contrast agent, and provide the date of your last scan.
You should not receive gadoterate meglumine if you are allergic to it.
Tell your doctor if you have ever had:
- kidney problems;
- any type of reaction to a contrast agent;
- diabetes;
- high blood pressure;
- asthma, hay fever, food or drug allergies;
- an injury, surgery, or severe infection; or
- if you are over 60 years old.
It is not known whether this medicine will harm an unborn baby. Tell your doctor if you are pregnant.
It may not be safe to breast-feed shortly after receiving this medicine. Ask your doctor about any risk.
Gadoterate meglumine Side Effects
Get emergency medical help if you have signs of an allergic reaction: hives; difficult breathing; swelling of your face, lips, tongue, or throat.
Some side effects of gadoterate meglumine may occur up to several days after injection.
Gadoterate meglumine can cause a life-threatening condition in people with advanced kidney disease. Call your doctor right away if you have any symptoms of this condition, such as:
- burning, itching, swelling, scaling, and tightening or hardening of your skin;
- muscle weakness;
- joint stiffness in your arms, hands, legs, or feet;
- deep bone pain in your ribs or your hips;
- trouble moving; or
- skin redness or discoloration.
Also call your doctor at once if you have:
- kidney problems–little or no urinating, painful or difficult urination, swelling in your feet or ankles, feeling tired or short of breath.
Common side effects of gadoterate meglumine may include:
- headache;
- nausea;
- rash; or
- pain or cold feeling around the IV needle.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Drug interaction
Other drugs may affect gadoterate meglumine, including prescription and over-the-counter medicines, vitamins, and herbal products. Tell your doctor about all your current medicines and any medicine you start or stop using.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.