Eptifibatide CAS NO 148031-34-9 Inquire about Eptifibatide
Tecoland supplies Eptifibatide bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Eptifibatide is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Eptifibatide?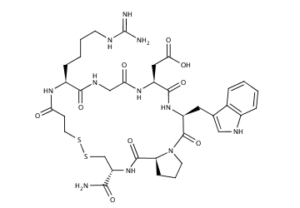
Eptifibatide (Integrilin, Millennium Pharmaceuticals, also co-promoted by Schering-Plough/Essex), is an antiplatelet drug of the glycoprotein IIb/IIIa inhibitor class. Eptifibatide is a cyclic heptapeptide derived from a protein found in the venom of the southeastern pygmy rattlesnake (Sistrurus miliarius barbouri). It belongs to the class of the so-called arginin-glycin-aspartat-mimetics and reversibly binds to platelets. Eptifibatide has a short half-life. The drug is the third inhibitor of GPIIb/IIIa that has found broad acceptance after the specific antibody abciximab and the non-peptide tirofiban entered the global market.
Eptifibatide is used to reduce the risk of acute cardiac ischemic events (death and/or myocardial infarction) in patients withunstable angina or non-ST-segment-elevation (e.g., non-Q-wave) myocardial infarction (i.e., non-ST-segment elevation acute coronary syndromes) both in patients who are to receive non surgery (conservative) medical treatment and those undergoing percutaneous coronary intervention (PCI).
The drug is usually applied together with aspirin or clopidogrel and (low molecular weight or unfractionated) heparin. Additionally, the usual supportive treatment consisting of applications of nitrates, beta-blockers, opioid analgesics and/or benzodiazepines should be employed as indicated. Angiographic evaluation and other intensive diagnostic procedures may be considered a first line task before initiating therapy with eptifibatide.
The drug should exclusively be used in hospitalized patients both because of the serious degree of patients’ illness and because of the possible side-effects of eptifibatide.
Side Effects
It should be noticed that all patients receiving eptifibatide were seriously ill and most of them were concomitantly treated with other drugs known to have the potential to cause significant side effects. Therefore, not all side effects listed as follows may be attributable to eptifibatide treatment alone:
The major adverse event in the PURSUIT study was severe bleeding. Bleeding occurred as well at sites of clinical intervention (local sites) as at other sites (systemically) like urogenital bleedings. Sometimes, these events were severe enough to require transfusion of blood or plasma concentrates to stop bleeding and counteract anemia. Severe bleedings occurred in 4.4 and 4.7% of patients respectively depending on the infusion rate (0.5 µg/kg/min vs. 0.75 µg/kg/min). A few cases of death due to severe bleeding events attributable to drug therapy were reported. No cases of hemorrhagic stroke were seen. Thrombocytopenia of unknown origin (allergic reaction?) was also noticed in 0.2% of patients.
Additionally, hypotension was seen frequently (6%). Cardiovascular failure was also frequent (2%) as were serious arrhythmias (ventricular fibrillation 1.5%, atrial fibrillation 6%). Severe allergic (anaphylactic) reactions occurred in almost 0.2% of patients. These reactions can be life-threatening and may be due to the peptide character of eptifibatide. Other side effects were rare and mild in nature and may not be connected to eptifibatide therapy.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.