Epinastine HCl CAS NO 108929-04-0 Inquire about Epinastine HCl
Tecoland supplies Epinastine HCl bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Epinastine HCl is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Epinastine HCl?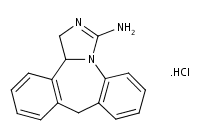
Epinastine HCl is an Ophthalmic solution used to prevent itching of the eyes caused by allergic conjunctivitis (a condition in which the eyes become itchy, swollen, red, and teary when they are exposed to certain substances in the air). Epinastine is in a class of medications called antihistamines.
How does Epinastine HCl work?
Epinastine HCl works by preventing the release of natural substances which cause allergic reactions in the eyes.
How should I use Epinastine HCl?
Epinastine comes as an ophthalmic solution (eye drops) to apply to the eyes. It is usually applied twice a day. To help you remember to use Epinastine eye drops, use them around the same times every day, usually morning and evening. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand. Use Epinastine eye drops exactly as directed. Do not use more or less of them or use them more often than prescribed by your doctor.
Epinastine eye drops are only for use in the eyes. Do not swallow this medication.
Epinastine eye drops control the itching of allergic conjunctivitis only when they are used regularly. Epinastine eye drops will not work if you use them only when you experience symptoms. Continue to use Epinastine eye drops even if you feel well. Do not stop using Epinastine eye drops without talking to your doctor.
When you apply Epinastine eye drops, be careful not to let the tip of the bottle touch your eye, fingers, face, or any surface. If the tip does touch another surface, bacteria may get into the eye drops. Using eye drops that are contaminated with bacteria may cause serious damage to the eye or loss of vision. If you think your eye drops have become contaminated, call your doctor or pharmacist.
To use the eye drops, follow these steps:
- Wash your hands thoroughly with soap and water.
- Use a mirror or have someone else put the drops in your eye.
- Remove the protective cap. Make sure that the end of the dropper tip is not chipped or cracked.
- Avoid touching the dropper tip against your eye, face, nose, fingers, or anything else.
- Hold the bottle with the tip down at all times to prevent drops from flowing back into the bottle and contaminating the medication inside.
- Lie down and gaze upward or tilt your head back.
- Holding the bottle between your thumb and index finger, place the dropper tip as near as possible to your eyelid without touching it.
- Brace the remaining fingers of that hand against your cheek or nose.
- With the index finger of your other hand, pull the lower lid of the eye down to form a pocket.
- Drop the prescribed number of drops into the pocket made by the lower lid and the eye. Placing drops on the surface of the eyeball can cause stinging.
- Close your eye and press lightly against the lower lid with your finger for 2-3 minutes to keep the medication in the eye. Do not blink.
- Repeat steps 7-11 above for your other eye.
- Replace the cap on the bottle and tighten it right away. Do not wipe or rinse off the tip.
What should I avoid while using Epinastine HCl?
- your prescriber will usually organize treatment for up to eight weeks
- if you wear contact lenses you should check with your prescriber if you can use this medicine while wearing contact lenses
- if you are using other eye medicines do not use one immediately after each other. This is to stop the second medicine from washing the first medicine out of the eye. You should find out from your prescriber how long you need to wait before using the second medicine
- make sure that you dispose of any unused medicine as detailed on the pharmacy label or in the patient information leaflet that comes with this medicine. If you are not sure, speak to your prescriber or pharmacist.
What are the possible side effects of Epinastine HCl?
Epinastine eye drops may cause side effects. It may be hard to tell if the symptoms you experience are side effects of Epinastine eye drops or are caused by allergies. Tell your doctor if any of these symptoms are severe or do not go away:
- burning or itchy eyes
- swollen eyelids
- eye redness
- headache
- runny nose
- cough
Some side effects can be serious. The following symptoms are uncommon, but if you experience either of them, call your doctor immediately:
- sore throat
- fever, chills, and other signs of infection
What other drugs will affect Epinastine HCl?
There are no known important interactions between Epinastine HCl and other medicines. If you experience any unusual symptoms while using Epinastine HCl and other medicines you should tell your prescriber.
Where can I get more information?
If you have questions about the medicine you are taking or would like more information, check with your doctor, pharmacist, or other health care provider
Epinastine HCl Storage
Keep this medication in the container it came in, tightly closed, and out of reach of children. Store it at room temperature and away from excess heat and moisture (not in the bathroom). Throw away any medication that is outdated or no longer needed. Talk to your pharmacist about the proper disposal of your medication.
Nursing Mothers use of Epinastine HCl
Epinastine is excreted in the breast milk of rats, but it is not known if Epinastine is excreted in human milk. Due to the lack of experience, caution should be exercised when prescribing to breast-feeding women.
Pregnancy and use of Epinastine HCl
Data on a limited number (11) of exposed pregnancies indicate no adverse effects of Epinastine on pregnancy or on the health of the fetus/newborn child. To date, no other relevant epidemiological data are available. Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryonic/fetal development, parturition or postnatal development. Caution should be exercised when prescribing to pregnant women.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.