Entacapone CAS NO 130929-57-6 Inquire about Entacapone
Tecoland supplies Entacapone bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Entacapone is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Entacapone?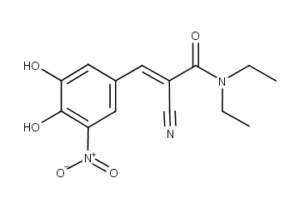
Entacapone is a drug that functions as a catechol-O-methyl transferase (COMT) inhibitor. It is used in the treatment of Parkinson’s disease. Entacapone is a member of the class of drugs known as nitrocatechols.
What is the mechanism of action?
When administered in conjunction with dopaminergic agents such as L-DOPA, entacapone prevents COMT from metabolizing L-DOPA into 3-methoxy-4-hydroxy-L-phenylalanine (3-OMD) in the periphery, which does not easily cross the blood brain barrier (BBB). Pharmacologically, entacapone is somewhat similar to carbidopa or benserazide, in that it is an inhibitor of an enzyme that converts L-DOPA into a compound that cannot cross the blood brain barrier. Carbidopa and benserazide inhibit aromatic L-amino acid decarboxylase, which converts L-DOPA into dopamine, which cannot cross the blood brain barrier.
Side Effects
The most frequent undesirable effects caused by entacapone relate to the increased effects of L-DOPA, such as involuntary movements (dyskinesias). These occur most frequently at the beginning of entacapone treatment. Others common side effects are gastrointestinal problems, including nausea and abdominal pains. Diarrhea is a frequently reported and troublesome side effect that can result in unnecessary investigation, but resolves quickly on withdrawal of the drug. Entacapone may cause urine to turn reddish-brown. This is a harmless side effect and is not a cause for concern. In studies with entacapone, some people have reported experiencing a dry mouth.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.