Degarelix acetate CAS NO 214766-78-6 Inquire about Degarelix acetate
Tecoland supplies Degarelix Acetate bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Degarelix Acetate is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Degarelix Acetate?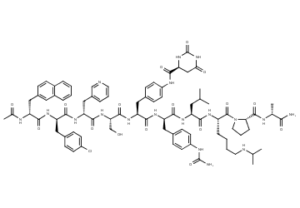
Degarelix or degarelix acetate (tradename Firmagon) is a hormonal therapy used in the treatment of prostate cancer. During development it was known as FE200486.
Testosterone is a male hormone that promotes growth of many prostate tumours and therefore reducing circulating testosterone to very low (castration) levels is often the treatment goal in the management of men with advanced prostate cancer. Degarelix has an immediate onset of action, binding to gonadotropin-releasing hormone (GnRH) receptors in the pituitary gland and blocking their interaction with GnRH. This induces a fast and profound reduction in luteinising hormone(LH), follicle-stimulating hormone (FSH) and in turn, testosterone suppression
Mechanism of action
GnRH antagonists (receptor blockers) such as degarelix are a new type of hormonal therapy for prostate cancer. These agents are synthetic peptide derivatives of the natural GnRH decapeptide – a hormone that is made by neurons in the hypothalamus. GnRH antagonists compete with natural GnRH for binding to GnRH receptors in the pituitary gland. This reversible binding blocks the release of LH and FSH from the pituitary. The reduction in LH subsequently leads to a rapid and sustained suppression of testosterone release from the testes and subsequently reduces the size and growth of the prostate cancer. This in turn results in a reduction in prostate-specific antigen (PSA) levels in the patient’s blood. Measuring PSA levels is a way to monitor how patients with prostate cancer are responding to treatment.
Unlike the GnRH agonists, which cause an initial stimulation of the hypothalamic-pituitary-gonadal axis (HPGA), leading to a surge in testosterone levels, and under certain circumstances, a flare-up of the tumour, GnRH antagonists do not cause a surge in testosterone or clinical flare. Clinical flare is a phenomenon that occurs in patients with advanced disease, which can precipitate a range of clinical symptoms such as bone pain, urethral obstruction, and spinal cord compression. Drug agencies have issued boxed warnings regarding this phenomenon in the prescribing information for GnRH agonists. As testosterone surge does not occur with GnRH antagonists, there is no need for patients to receive an antiandrogen as flare protection during prostate cancer treatment. GnRH agonists also induce an increase in testosterone levels after each reinjection of the drug – a phenomenon that does not occur with GnRH antagonists such as degarelix.
GnRH antagonists have an immediate onset of action leading to a fast and profound suppression of testosterone and are therefore especially valuable in the treatment of patients with prostate cancer where fast control of disease is needed.
Side Effects
As with all hormonal therapies, degarelix is commonly associated with hormonal side effects such as hot flashes and weight gain. Due to its mode of administration (subcutaneous injection), degarelix is also associated with injection-site reactions such as injection-site pain, erythema or swelling. Injection-site reactions are usually mild or moderate in intensity and occur predominantly after the first dose, decreasing in frequency thereafter.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.