Caspofungin Acetate CAS NO 179463-17-3 Inquire about Caspofungin Acetate
Tecoland supplies Caspofungin bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Caspofungin is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Caspofungin Acetate?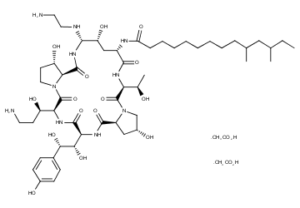
Caspofungin Acetate is a sterile, lyophilized product for intravenous (IV) infusion that contains a semisynthetic lipopeptide (echinocandin) compound synthesized from a fermentation product of Glarea lozoyensis.
Caspofungin acetate is an echinocandin that inhibits the synthesis of ? (1,3)-D-glucan, an integral component of the fungal cell wall.
Caspofungin acetate is 1-[(4R,5S)-5-[(2-aminoethyl)amino]-N2-(10,12-dimethyl-1oxotetradecyl)-4-hydroxy-L-ornithine]-5-[(3R)-3-hydroxy-L-ornithine] pneumocandin B0 diacetate (salt). Caspofungin acetate 50 mg also contains: 39 mg sucrose, 26 mg mannitol, glacial acetic acid, and sodium hydroxide. Caspofungin acetate 70 mg also contains 54 mg sucrose, 36 mg mannitol, glacial acetic acid, and sodium hydroxide. Caspofungin acetate is a hygroscopic, white to off-white powder. It is freely soluble in water and methanol, and slightly soluble in ethanol. The pH of a saturated aqueous solution of caspofungin acetate is approximately 6.6. The empirical formula is C52H88N10O15·2C2H4O2 and the formula weight is 1213.42.
Indications
Caspofungin acetate for injection was originally approved by both the Food and Drug Administration (FDA), in the US, and the EMEA, in Europe, in 2001. Its currently approved therapeutic indications by both organisations include the empirical therapy of presumed fungal infections in febrile, neutropenic adult patients and the treatment of invasive aspergillosis in adult patients whose disease is refractory to, or who are intolerant of, other antifungal agents (i.e., conventional or lipid formulations of amphotericin B and/or itraconazole). Additionally, the FDA approval includes indication for the treatment of candidemia and some specific Candida infections (intra-abdominal abscesses, peritonitis, pleural cavity infections and oesophagitis) and the EMEA approval includes indication for the treatment of general invasive candidiasis in adult patients.
Clinical efficacy
About 36% of patients refractory to other therapies responded well to caspofungin therapy, while even 70% of patients intolerant to other therapies were classified as responders. Direct comparative studies to other drugs in the treatment of invasive aspergillosis have so far not been undertaken.
Molecular Formula C52H88N10O15·2C2H4O2
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.