Olmesartan Medoxomil CAS NO 144689-63-4 Inquire about Olmesartan Medoxomil
Tecoland supplies Olmesartan Medoxomil bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Olmesartan Medoxomil is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Olmesartan Medoxomil?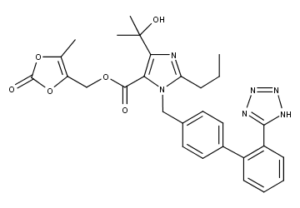
Olmesartan medoxomil (trade names: Benicar in the US, Olmetec in EU and Canada, WinBP, Golme in India, Erastapex in Egypt) is an angiotensin II receptor antagonist used to treat high blood pressure.
Indications
Olmesartan is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents. The U.S. Food and Drug Administration (FDA) has determined that the benefits of Benicar continue to outweigh its potential risks when used for the treatment of patients with high blood pressure according to the drug label.
Contraindications
Contraindications for treatment with olmesartan include biliary obstruction (BNF).
Cautions
Angiotensin-II receptor antagonists should be used with caution in renal artery stenosis. Monitoring of plasma-potassium concentration is advised, particularly in the elderly and in patients with renal impairment; lower initial doses may be appropriate in these patients. Angiotensin-II receptor antagonists should be used with caution in aortic or mitral valve stenosis and in hypertrophic cardiomyopathy. Those with primary aldosteronism, and Afro-Caribbean patients (particularly those with left ventricular hypertrophy), may not benefit from an angiotensin-II receptor antagonist.
Adverse effects
The incidence of adverse effects with BENICAR (the trade name for olmesartan medoxomil) is reported as similar to placebo; the only adverse effect that occurred in >1% of patients treated with BENICAR and more frequently than placebo was dizziness (3% vs 1%). The full prescribing information for Benicar notes that as with all drugs that act directly on the renin-angiotensin system, olmesartan is contraindicated in pregnancy and can cause injury and even death to the developing fetus. In studies of angiotensin II receptor antagonists such as olmesartan, patients with unilateral or bilateral renal artery stenosis, increases in serum creatinine or blood urea nitrogen (BUN) have been reported. There has been no long-term use of olmesartan medoxomil in patients with unilateral or bilateral renal artery stenosis, but similar results may be expected.
Structure
The olmesartan molecule includes one tetrazole group (a 5-member heterocyclic ring of four nitrogen and one carbon atom) and one imidazole group (a 5-membered planar heterocyclic aromatic ring of two nitrogen and three carbon atoms, classified as an alkaloid).
Mechanism of action
Olmesartan is a prodrug that works by blocking the binding of angiotensin II to the AT1 receptors in vascular muscle; it is therefore independent of angiotensin II synthesis pathways, unlike ACE inhibitors. By blocking the binding rather than the synthesis of angiotensin II, olmesartan inhibits the negative regulatory feedback on renin secretion. As a result of this blockage, olmesartan reduces vasoconstriction and the secretion of aldosterone. This lowers blood pressure by producing vasodilation, and decreasing peripheral resistance.
Interactions
Do not use non-prescription products that contain stimulants; including diet pills, and cold medicines. Consult your doctor before taking any potassium supplements, including salt substitutes.
Dosage and administration
The usual recommended starting dose of olmesartan is 20 mg once daily. The dose may be increased to 40 mg after 2 weeks of therapy, if further reduction in blood pressure is desirable. Doses above 40 mg do not appear to have greater effect, and twice-daily dosing offers no advantage over the same total dose given once daily.No adjustment of dosage is typically necessary for advanced age, renal impairment, or hepatic dysfunction. For patients with possible depletion of intravascular volume (e.g., patients treated with diuretics), olmesartan should be initiated with caution; consideration should be given to use of a lower starting dose in such cases.If blood pressure is not controlled by Benicar alone, a diuretic may be added. Benicar may be administered with other antihypertensive agents. Benicar may be administered with or without food.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.