Rezafungin CAS NO 1396640-59-7 Inquire about Rezafungin
Tecoland supplies Rezafungin bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Rezafungin is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Rezafungin 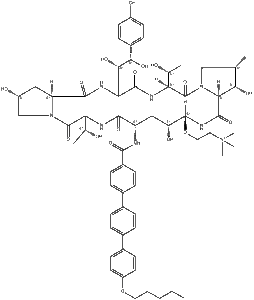
Rezafungin is under investigation in clinical trial NCT04368559 (Study of Rezafungin Compared to Standard Antimicrobial Regimen for Prevention of Invasive Fungal Diseases in Adults Undergoing Allogeneic Blood and Marrow Transplantation).
Rezafungin is a novel echinocandin being developed for treatment of candidemia and invasive candidiasis and for prevention of invasive fungal disease caused by Candida, Aspergillus, and Pneumocystis spp. in recipients of blood and marrow transplantation.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.