Carmustine CAS NO 154-93-8 Inquire about Carmustine
Tecoland supplies Carmustine bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Carmustine is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Carmustine 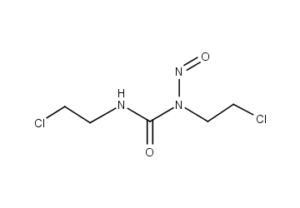
Carmustine is used to treat brain tumors, Hodgkin’s disease, multiple myeloma, and non-Hodgkin’s lymphoma.
Carmustine is sometimes given with other cancer medicines, with radiation or after brain surgery.
Carmustine may also be used for purposes not listed in this medication guide.
Before taking this medicine
You should not be treated with carmustine if you are allergic to it.
Tell your doctor if you have ever had:
- lung disease or breathing problems;
- bone marrow suppression; or
- kidney disease.
Receiving carmustine injection may increase your risk of developing other cancers, such as leukemia. Ask your doctor about this risk.
Carmustine may harm an unborn baby if the mother or the father is using this medicine.
- If you are a woman, do not use carmustine if you are pregnant. Use effective birth control to prevent pregnancy while receiving carmustine. Keep using birth control for at least 6 months after your last injection or after implant placement.
- If you are a man, use effective birth control if your sex partner is able to get pregnant. Keep using birth control for at least 3 months after your last injection or after implant placement.
- Tell your doctor right away if a pregnancy occurs while either the mother or the father is using carmustine.
This medicine may affect fertility (ability to have children) in men. However, it is important to use birth control to prevent pregnancy because carmustine can harm an unborn baby.
Do not breastfeed while receiving carmustine injection, or for at least 7 days after carmustine implant placement.
Carmustine Side Effects
Get emergency medical help if you have signs of an allergic reaction: hives; difficult breathing; swelling of your face, lips, tongue, or throat.
You may have an infusion reaction during the injection or within 2 hours afterward. This may include skin redness, eye redness and severe warmth or tingling under your skin.
Some side effects may not occur many weeks or even years after you receive carmustine.
Carmustine may cause serious side effects. Call your doctor at once if you have:
- easy bruising, unusual bleeding;
- a seizure;
- unexplained weight loss;
- little or no urination; or
- pain, burning, swelling, or skin changes where the injection was given;
- slow healing of your incision after carmustine implant placement;
- lung problems–a dry cough or hack, shortness of breath (especially with exercise), rapid but shallow breathing, tiredness, body aches, clubbing (widening and rounding) of your fingertips or toes;
- increased pressure inside your skull–sudden vision problems, severe headache, vomiting, dizziness; or
- signs of meningitis–fever, neck stiffness, increased sensitivity to light, nausea, vomiting, confusion, drowsiness.
Common side effects of carmustine may include:
- bleeding, bruising;
- tiredness;
- nausea, vomiting; or
- breathing problems.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Carmustine side effects (more detail)
Drug Interaction
Other drugs may affect carmustine, including prescription and over-the-counter medicines, vitamins, and herbal products. Tell your doctor about all your current medicines and any medicine you start or stop using.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.