Lifitegrast CAS NO 1025967-78-5 Inquire about Lifitegrast
Tecoland supplies Lifitegrast bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Lifitegrast is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Lifitegrast?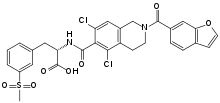
Lifitegrast (trade name Xiidra) is an FDA approved drug indicated for the treatment of signs and symptoms of dry eye, a syndrome called keratoconjunctivitis sicca. Lifitegrast reduces inflammation by inhibiting inflammatory cell binding. It is often used in conjunction with ciclosporin (Ikervis or Restasis) for dry eye treatment including meibomian gland dysfunction and inflammatory dry eye.
Mechanism of Action:
Lifitegrast inhibits an integrin, lymphocyte function-associated antigen 1 (LFA-1), from binding to intercellular adhesion molecule 1 (ICAM-1). This mechanism down-regulates inflammation mediated by T lymphocytes.
Pharmacology:
Lifitegrast is supplied as an eye drop and typically applied two times a day.
Lifitegrast Side effects:
Common side effects in clinical trials were eye irritation, discomfort, blurred vision, and dysgeusia.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.