Mirabegron CAS NO 223673-61-8 Inquire about Mirabegron
Tecoland supplies Mirabegron bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Mirabegron is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Mirabegron?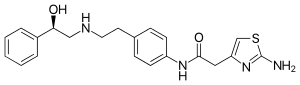
Mirabegron is a drug for the treatment of overactive bladder. It was approved in the United States in July 2012.
Mirabegron has also recently been shown to activate brown fat and increase metabolism. In a small study of 15 healthy, lean men, mirabegron was shown to increase basal heart rate, metabolic rate and blood pressure which are signs of cardiovascular stimulation.
Side Effects
The most common side effect is elevated blood pressure, other side effects include dry mouth, nasopharyngitis, UTI, headache, constipation, etc.
Mechanism of action
Mirabegron activates the β3 adrenergic receptor in the detrusor muscle in the bladder, which leads to muscle relaxation and an increase in bladder capacity
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.