Tedizolid CAS NO 856867-55-5 Inquire about Tedizolid
Tecoland supplies Tedizolid bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Tedizolid is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Tedizolid?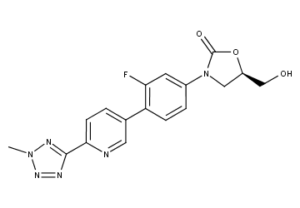
Tedizolid Phosphate (formerly torezolid phosphate, TR-701), marketed as the phosphate salt under the trade name Sivextro, is an oxazolidinone-class antibiotic developed by Cubist Pharmaceuticals, following acquisition of Trius Therapeutics (originator: Dong-A Pharmaceuticals), for the treatment of acute bacterial skin and skin structure infections (also known as complicated skin and skin-structure infections (cSSSIs)).
Sivextro has been approved by the U.S Food and Drug Administration on June 20, see 2014, for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by certain susceptible bacteria, including Staphylococcus aureus (including methicillin-resistant strains (MRSA) and methicillin-susceptible strains), various Streptococcus species (Streptococcus pyogenes, seek Streptococcus agalactiae, Streptococcus anginosus Group (including Streptococcus anginosus, Streptococcus intermedius, and Streptococcus constellatus)), and Enterococcus faecalis. Tedizolid is a second-generation oxazolidinone that is 4-to-16-fold more potent against staphylococci and enterococci compared to linezolid. The recommended dosage for treatment is 200 mg once daily for a total duration of six days, either orally (with or without food) or through an intravenous injection (if patient is older than 18 years old).
Mechanism of action
Tedizolid phosphate is a prodrug activated by plasma phosphatases to tedizolid following administration of the drug either orally or intravenously. The prodrug tedizolid is called “TR-701”, while the active moiety is called “TR-700”. Once activated, tedizolid exerts its bacteriostatic microbial activity through inhibition of protein synthesis by binding to the 50S ribosomal subunit of the bacteria.
Adverse effects
The most common adverse effects found in the clinical trials were nausea, headache, diarrhea, vomiting, and dizziness. Sivextro has also been found to have hematologic effects, as shown in Phase-I studies in which subjects exposed to doses longer than 6 days showed a possible dose and duration effect on hematologic parameters. Its safety in patients with decreased levels of white blood cells has not been established; thus, alternative treatments should be considered. Patients on tedizolid are also at low risk of peripheral and optic neuropathy, similar to other members of the oxazolidinone class.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.