Sulfasalazine CAS NO 599-79-1 Inquire about Sulfasalazine
Tecoland supplies Sulfasalazine bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Sulfasalazine is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Sulfasalazine?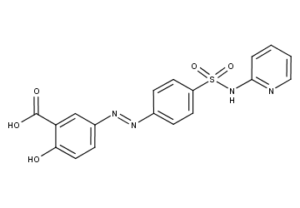
Sulfasalazine (brand name Azulfidine in the U.S, Salazopyrin and Sulazine in Europe and Hong Kong) was developed in the 1950s specifically to treat rheumatoid arthritis. It was believed at the time that bacterial infections were the cause of rheumatoid arthritis. Sulfasalazine is a sulfa drug, (a derivative of mesalazine) and is formed by combining sulfapyridine and salicylate with an azo bond. It may be abbreviated SSZ.
Indications
Sulfasalazine is used in the treatment of inflammatory bowel disease, including ulcerative colitis and Crohn’s disease. It is also indicated for use in rheumatoid arthritis and used in other types of inflammatory arthritis (e.g. psoriatic arthritis) where it has a beneficial effect. It is often well tolerated compared to other DMARDS.
In clinical trials for the treatment of chronic alcoholics, sulfasalazine has been found to reverse the scarring associated with cirrhosis of the liver. Cells called myofibroblasts, which contribute to scar tissue in a diseased liver, also appear to secrete proteins that prevent the breakdown of the scar tissue. Sulfasalazine appears to retard this secretion.
A study at University of Newcastle found that the drug may also act to aid the healing of cirrhosis of the liver.
It is usually not given to children under 2 years of age.
The use of sulfasalazine in inflammatory bowel disease has declined due mainly to the fact that it yields the metabolite sulfapyridine which gives rise to side-effects such as agranulocytosis and hypospermia. However, the other metabolite of sulfasalazine, 5-aminosalicylic acid (5-ASA) is attributed to the drug’s therapeutic effect. Therefore, 5-ASA and other derivatives of 5-ASA, are now usually preferred and given alone (as mesalazine), despite their increased cost, due to their more favorable side-effect profile.
Sulfasalazine has also been used successfully to treat cases of idiopathic urticaria that do not respond to antihistamines.
What is the mechanism of action?
The mode of action of Sulfasalazine or its metabolites, 5-aminosalicylic acid (5-ASA) and sulfapyridine (SP), is still under investigation, but may be related to the anti-inflammatory and/or immunomodulatory properties that have been observed in animal and in vitro models, to its affinity for connective tissue, and/or to the relatively high concentration it reaches in serous fluids, the liver and intestinal walls, as demonstrated in autoradiographic studies in animals. In ulcerative colitis, clinical studies utilizing rectal administration of Sulfasalazine, SP and 5-ASA have indicated that the major therapeutic action may reside in the 5-ASA moiety. The relative contribution of the parent drug and the major metabolites in rheumatoid arthritis is unknown.
Precautions
Before taking sulfasalazine,
- tell your doctor if you are allergic to sulfasalazine, sulfapyridine, aspirin, choline magnesium trisalicylate (Triosal, Trilisate), choline salicylate (Arthropan), mesalamine (Asacol, Pentasa, Rowasa), salsalate (Argesic-SA, Disalcid, Salgesic, others), sulfa drugs, trisalicylate (Tricosal, Trilisate),or any other drugs.
- tell your doctor and pharmacist what prescription and nonprescription medications you are taking, especially digoxin (Lanoxin), folic acid, and vitamins.
- tell your doctor if you have or have ever had asthma, kidney or liver disease, porphyria, blood problems, or blockage in your intestine or urinary tract.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while taking sulfasalazine, call your doctor.
- plan to avoid unnecessary or prolonged exposure to sunlight and to wear protective clothing, sunglasses, and sunscreen. Sulfasalazine may make your skin sensitive to sunlight.
Usage and dosage of Sulfasalazine
Sulfasalazine comes as regular and delayed-release (enteric-coated) tablets. It usually is taken four times a day in evenly spaced doses throughout the day so that no more than 8 hours separates any two doses, if possible. Take sulfasalazine after a meal or with a light snack, and then drink a full glass of water. Follow the directions on your prescription label carefully. Take sulfasalazine exactly as directed. Do not take more or less of it or take it more often than prescribed by your doctor.
- Swallow tablets whole; do not crush or chew them.
- Drink plenty of fluids (at least six to eight glasses of water or other beverage per day) while taking sulfasalazine.
- Continue to take sulfasalazine even if you feel well. Do not stop taking sulfasalazine without talking to your doctor.
- May take Vitamin D.
- Take with a full glass of water. No iron, zinc or fluoride within 2 hours of taking this medication.
- Take sulfasalazine with food.
What are the side effects of Sulfasalazine?
Sulfsalazine metabolizes to sulfapyridine. Serum levels should be monitored every three months, and more frequently at the outset. Serum levels above 50¦Ìg/l are associated with side effects. In rare cases, Sulfasalazine can cause severe depression in young males. It can also cause temporary infertility. Immune thrombocytopenia has been reported.
Sulfasalazine inhibits dihydrofolate reductase, and can cause folate deficiency and megaloblastic anemia.
Sulfasalazine can cause hemolytic anemia in people with G6PD deficiency.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.