Felodipine CAS NO 72509-76-3 Inquire about Felodipine
Tecoland supplies Felodipine bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Felodipine is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Felodipine?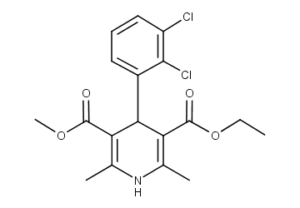
Felodipine is a calcium channel blocker (calcium antagonist), a drug used to control hypertension (high blood pressure). It is marketed under the brand name Plendil by AstraZeneca and Renedil by Sanofi-Aventis. The formulation patent for the substance expired in 2007.
AstraZeneca dropped Plendil from its support and AZ&Me free Rx access program in October 2008.
Interactions
Febuxostat is a non-purine selective inhibitor of xanthine oxidase. It works by non-competitively blocking the molybdenum pterin center which is the active site on xanthine oxidase. Xanthine oxidase is needed to successively oxidize both hypoxanthine and xanthine to uric acid. Hence, febuxostat inhibits xanthine oxidase, therefore reducing production of uric acid. Febuxostat inhibits both, oxidized as well as reduced form of xanthine oxidase because of which febuxostat cannot be easily displaced from the molybdenum pterin site.
Clinical Efficacy
Many long and short-term clinical trials have proved the efficacy of Febuxostat in the treatment of gout and lowering uric acid levels. In these studies Febuxostat was found to be superior to Allopurinol in reducing the serum uric acid levels. Some notable landmark clinical trials are FACT, APEX, EXCEL, FOCUS and CONFIRMS.
Febuxostat versus Allopurinol Controlled Trial (FACT): Serum urate levels were reduced below 6.0 mg/dL at the last three monthly observations in a significantly greater proportion of patients with gout and hyperuricaemia receiving febuxostat 80 or 120 mg once daily than in those receiving allopurinol 300 mg once daily in a 52-week, randomized, double-blind trial.
Allopurinol Placebo controlled Efficacy study of febuXostat (APEX): Febuxostat 80, 120 or 240 mg once daily showed significantly greater urate-lowering efficacy than allopurinol 100 or 300 mg once daily in a 28-week, randomized, double-blind, placebo-controlled trial in patients with gout and hyperuricaemia.
Side effect?
The adverse effects associated with febuxostat therapy include nausea, diarrhea, arthralgia, headache, increased hepatic serum enzyme levels and rash.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.