Alogliptin Benzoate CAS NO 850649-62-6 Inquire about Alogliptin Benzoate
Tecoland supplies Alprostadil bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Alprostadil is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Alogliptin Benzoate?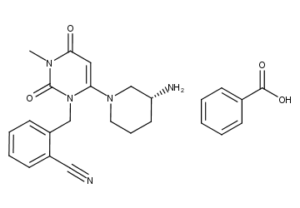
Alogliptin benzoate is a white to off-white, crystalline powder containing one asymmetric carbon in the aminopiperidine moiety. It is soluble in dimethylsulfoxide, sparingly soluble in water and methanol, slightly soluble in ethanol, and very slightly soluble in octanol and isopropyl acetate.
Each NESINA tablet contains 34 mg, 17 mg, or 8.5 mg alogliptin benzoate which is equivalent to 25 mg, 12.5 mg, or 6.25 mg, respectively, of alogliptin and the following inactive ingredients: mannitol, microcrystalline cellulose, hydroxypropyl cellulose, croscarmellose sodium, and magnesium stearate. In addition, the film-coating contains the following inactive ingredients: hypromellose, titanium dioxide, ferric oxide (red or yellow), and polyethylene glycol, and is marked with printing ink (Gray F1).
Mechanism of Action
Increased concentrations of the incretin hormones such as glucagon-likepeptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are released into the bloodstream from the small intestine in response to meals. These hormones cause insulin release from the pancreatic beta cells in a glucose-dependent manner but are inactivated by the DPP-4 enzyme within minutes. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, reducing hepatic glucose production. In patients with type 2 diabetes, concentrations of GLP-1 are reduced but the insulin response to GLP-1 is preserved. Alogliptin is a DPP-4 inhibitor that slows the inactivation of the incretin hormones, thereby increasing their bloodstream concentrations and reducing fasting and postprandial glucose concentrations in a glucose-dependent manner in patients with type 2 diabetes mellitus. Alogliptin selectively binds to and inhibits DPP-4 but not DPP-8 or DPP-9 activity in vitro at concentrations approximating therapeutic exposures.
Pharmacodynamics
Single-dose administration of NESINA to healthy subjects resulted in a peak inhibition of DPP-4 within 2 to 3 hours after dosing. The peak inhibition of DPP-4 exceeded 93% across doses of 12.5 mg to 800 mg. Inhibition of DPP-4 remained above 80% at 24 hours for doses greater than or equal to 25 mg. Peak and total exposure over 24 hours to active GLP-1 were 3- to 4-fold greater with NESINA (at doses of 25 to 200 mg) than placebo. In a 16-week, double-blind, placebo-controlled study, NESINA 25 mg demonstrated decreases in postprandial glucagon while increasing postprandial active GLP-1 levels compared to placebo over an 8-hour period following a standardized meal. It is unclear how these findings relate to changes in overall glycemic control in patients with type 2 diabetes mellitus. In this study, NESINA 25 mg demonstrated decreases in 2-hour postprandial glucose compared to placebo (-30 mg/dL versus 17 mg/dL, respectively).
Multiple-dose administration of alogliptin to patients with type 2 diabetes also resulted in a peak inhibition of DPP-4 within 1 to 2 hours and exceeded 93% across all doses (25 mg, 100 mg, and 400 mg) after a single dose and after 14 days of once-daily dosing. At these doses of NESINA, inhibition of DPP-4 remained above 81% at 24 hours after 14 days of dosing.
Absorption
The absolute bioavailability of NESINA is approximately 100%. Administration of NESINA with a high-fat meal results in no significant change in total and peak exposure to alogliptin. NESINA may therefore be administered with or without food.
Distribution
Following a single, 12.5 mg intravenous infusion of alogliptin to healthy subjects, the volume of distribution during the terminal phase was 417 L, indicating that the drug is well distributed into tissues.
Alogliptin is 20% bound to plasma proteins.
Metabolism
Alogliptin does not undergo extensive metabolism and 60% to 71% of the dose is excreted as unchanged drug in the urine.
The (S)-enantiomer is not detectable at the 25 mg dose.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.