Tafluprost CAS NO 209860-87-7 Inquire about Tafluprost
Tecoland supplies Tafluprost bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Tafluprost is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Tafluprost?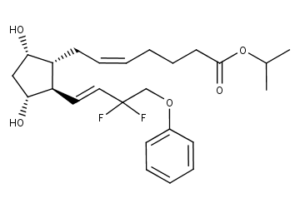
Tafluprost is a fluorinated analog of prostaglandin F2?. The chemical name for tafluprost is 1-methylethyl (5Z)-7-{(1R, 2R, 3R, 5S)-2-[(1E)-3, 3-difluoro-4-phenoxy-1-butenyl}-3, 5-dihydroxycyclopentyl]-5-heptenoate. The molecular formula of tafluprost is C25H34F2O5 and its molecular weight is 452.53.
Tafluprost is a colorless to light yellow viscous liquid that is practically insoluble in water.
Tafluprost is a prostaglandin analogue used topically (as eye drops) to control the progression of glaucoma and in the management of ocular hypertension. It reduces intraocular pressure by increasing the outflow of aqueous fluid from the eyes.
Precautions before using Tafluprost
Pigmentation
Tafluprost has been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the iris, periorbital tissue (eyelid) and eyelashes. Pigmentation is expected to increase as long as tafluprost is administered. The pigmentation change is due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes. After discontinuation of tafluprost, pigmentation of the iris is likely to be permanent, while pigmentation of the periorbital tissue and eyelash changes have been reported to be reversible in some patients. Patients who receive treatment should be informed of the possibility of increased pigmentation. The long term effects of increased pigmentation are not known.
Iris color change may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil spreads concentrically towards the periphery of the iris and the entire iris or parts of the iris become more brownish. Neither nevi nor freckles of the iris appear to be affected by treatment. While treatment with Tafluprost can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly.
Eyelash Changes
Tafluprost may gradually change eyelashes and vellus hair in the treated eye. These changes include increased length, color, thickness, shape and number of lashes. Eyelash changes are usually reversible upon discontinuation of treatment.
Intraocular Inflammation
Tafluprost should be used with caution in patients with active intraocular inflammation (e.g., iritis/uveitis) because the inflammation may be exacerbated.
Macular Edema
Macular edema, including cystoid macular edema, has been reported during treatment with prostaglandin F2? analogs. Tafluprost should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Tafluprost Side effects
Most common ocular adverse reaction is conjunctival hyperemia (range 4% – 20%).
Tafluprost Storage
Store refrigerated at 2 – 8°C (36 – 46°F).
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.