Ranitidine Bismuth Citrate CAS NO 128345-62-0 Inquire about Ranitidine Bismuth Citrate
Tecoland supplies Ranitidine Bismuth Citrate bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Ranitidine Bismuth Citrate is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Ranitidine Bismuth Citrate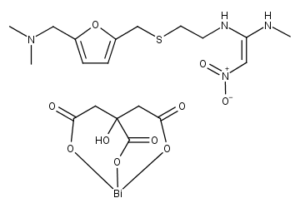
Ranitidine Bismuth Citrate is a member of the class of histamine H2-receptor antagonists with antacid activity. Ranitidine is a competitive and reversible inhibitor of the action of histamine, released by enterochromaffin-like (ECL) cells, at the histamine H2-receptors on parietal cells in the stomach, thereby inhibiting the normal and meal-stimulated secretion of stomach acid. In addition, other substances that promote acid secretion have a reduced effect on parietal cells when the H2 receptors are blocked.
Ranitidine bismuth citrate was withdrawn from the U.S. market in 1998.
Ranitidine is in a class of drugs called histamine receptor antagonists. Ranitidine works by decreasing the amount of acid your stomach produces.
Bismuth is a mild antibiotic.
Citrate is a form of salt.
Before taking this medicine
Before taking this medication, tell your doctor if you have
- kidney disease;
- liver disease; or
- acute porphyria.
You may not be able to take ranitidine bismuth citrate, or you may require a lower dose or special monitoring during treatment if you have any of the conditions listed above.
Ranitidine bismuth citrate is in the FDA pregnancy category C. This means that it is unknown whether ranitidine bismuth citrate will harm an unborn baby. Do not take ranitidine bismuth citrate without first talking to your doctor if you are pregnant.
It is also not known whether ranitidine bismuth citrate passes into breast milk. Do not take ranitidine bismuth citrate without first talking to your doctor if you are breast-feeding a baby.
Ranitidine bismuth citrate Side Effects
Stop taking ranitidine bismuth citrate and seek emergency medical attention if you experience an allergic reaction (difficulty breathing; closing of your throat; swelling of your lips, tongue, or face; or hives).
Other, less serious side effects may be more likely to occur. Continue to take ranitidine bismuth citrate and talk to your doctor if you experience
- changes in taste;
- headache or dizziness;
- diarrhea, nausea, or constipation; or
- tremor (shaking).
Side effects other than those listed here may also occur. Talk to your doctor about any side effect that seems unusual or that is especially bothersome.
Drug Interactions
Before taking ranitidine bismuth citrate, tell your doctor if you are taking any of the following medicines:
- Sedatives, sleeping pills, or tranquilizers such as alprazolam (Xanax), diazepam (Valium), and chlordiazepoxide (Librium) may cause dangerous sedation when taken with ranitidine bismuth citrate.
- Anticoagulants (blood thinners) such as warfarin (Coumadin) may have increased effects, which could result in bleeding.
- Seizure medications such as phenytoin (Dilantin) and carbamazepine (Tegretol) may have dangerous side effects when taken with ranitidine bismuth citrate.
- Medications for heart disorders, such as procainamide (Procanbid, Procan SR, Pronestyl), propranolol (Inderal), and metoprolol (Lopressor), may have increased effects on your heart when you are taking ranitidine bismuth citrate.
- Oral diabetes drugs such as glipizide (Glucotrol), glyburide (Diabeta, Micronase), and tolbutamide (Tolinase) may have increased effects, and very low blood sugar levels may result.
- Cisapride (Propulsid), which is taken for stomach conditions, as well as antifungal drugs such as ketoconazole (Nizoral), itraconazole (Sporanox), and fluconazole (Diflucan), may increase side effects.
Drugs other than those listed here may also interact with ranitidine bismuth citrate. Talk to your doctor and pharmacist before taking any prescription or over-the-counter medicines.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.