Quetiapine Fumarate CAS NO 111974-72-2 Inquire about Quetiapine Fumarate
Tecoland supplies Quetiapine Fumarate bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Quetiapine Fumarate is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Quetiapine fumarate?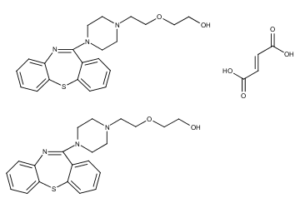
Quetiapine is an atypical antipsychotic approved for the treatment of schizophrenia, bipolar disorder, and along with an antidepressant to treat major depressive disorder.
Quetiapine is effective when used by itself and when used along with other medications in major depressive disorder(MDD). However, sedation is often an undesirable side effect.
In the US, the UK and Australia (in this indication it isn’t covered by the Pharmaceutical Benefits Scheme), quetiapine is licensed for use as an adjunct in the treatment of MDD.
Alzheimer’s disease
Quetiapine does not decrease agitation among people with Alzheimer’s. Quetiapine worsens intellectual functioning in the elderly with dementia and therefore is not recommended.
Other
The use of low doses of quetiapine for insomnia, while common, is not recommended; there is little evidence of benefit and concerns regarding adverse effects. It is sometimes used off-label, often as an augmentation agent, to treat conditions such as Tourette syndrome, musical hallucinations and anxiety disorders.
Quetiapine and clozapine are the most widely used medications for the treatment of Parkinson’s disease psychosis due to their very low extrapyramidal side effect liability. Owing to the risks associated with clozapine (e.g. agranulocytosis, diabetes mellitus, etc.), clinicians often attempt treatment with quetiapine first, although the evidence to support quetiapine’s use for this indication is significantly weaker than that of clozapine.
Adverse Effect
Very common (>10% incidence) adverse effects
- Dry mouth
- Dizziness
- Headache
Somnolence (drowsiness; of 15 antipsychotics quetiapine causes the 5th most sedation. Extended release (XR) formulations tend to produce less sedation, dose-by-dose than the immediate release formulations).
Both typical and atypical antipsychotics can cause tardive dyskinesia. According to one study, rates are lower with the atypicals at 3.9% as opposed to the typicals at 5.5%. Although Quetiapine and Clozapine are atypical antipsychotics, switching to these atypicals is an option to minimize symptoms of tardive dyskinesia caused by other atypicals.
Weight gain can be a problem for some, with quetiapine causing more weight gain than fluphenazine, haloperidol, loxapine, molindone, olanzapine, pimozide, risperidone, thioridazine, thiothixene, trifluoperazine, and ziprasidone, but less than chlorpromazine, clozapine, perphenazine, and sertindole.
Studies conducted on beagles have resulted in the formation of cataracts. While there are reports of cataracts occurring in humans, controlled studies including thousands of patients have not demonstrated a clear causal association between quetiapine therapy and this side-effect. However, the Seroquel website still recommends users have eye examinations every six months. As with some other anti-psychotics, quetiapine may lower the seizure threshold, and should be taken with caution in combination with drugs such as bupropion.
Overdose
Most instances of acute overdosage result only in sedation, hypotension and tachycardia, but cardiac arrhythmia, coma and death have occurred in adults. Serum or plasma quetiapine concentrations are usually in the 1–10 mg/L range in overdose survivors, while postmortem blood levels of 10–25 mg/L are generally observed in fatal cases. Non-toxic levels in postmortem blood extend to around 0.8 mg/kg, but toxic levels in postmortem blood can begin at 0.35 mg/kg.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.