Pomalidomide CAS NO 19171-19-8 Inquire about Pomalidomide
Tecoland supplies Pomalidomide bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Pomalidomide is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Pomalidomide?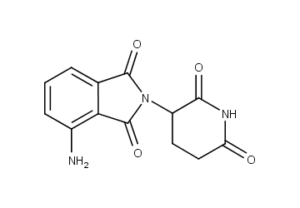
Pomalidomide (originally CC-4047 or 3-amino-thalidomide, trade name Pomalyst in the US and Imnovid in Europe) is a derivative of thalidomide marketed by Celgene. It is anti-angiogenic and also acts as an immunomodulator. Pomalidomide was approved in February 2013 by the U.S. Food and Drug Administration (FDA) as a treatment for relapsed and refractory multiple myeloma. It received a similar approval from the European Commission in August 2013.
Mechanism of action
Pomalidomide directly inhibits angiogenesis and myeloma cell growth. This dual effect is central to its activity in myeloma, rather than other pathways such as TNF alpha inhibition, since potent TNF alpha inhibitors including rolipram and pentoxifylline do not inhibit myeloma cell growth nor angiogenesis. Up regulation of Interferon gamma, IL-2 and IL-10 as well as down regulation of IL-6 have been reported for pomalidomide. These changes may contribute to pomalidomide’s anti-angiogenic and anti-myeloma activities.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.