Parecoxib Sodium CAS NO 198470-85-8 Inquire about Parecoxib Sodium
Tecoland supplies Parecoxib Sodium bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Parecoxib Sodium is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Parecoxib Sodium?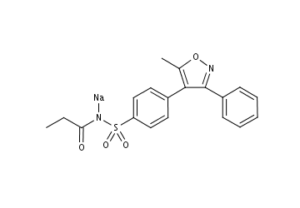
Parecoxib is a water-soluble and injectable prodrug of valdecoxib. It is marketed as Dynastat in the European Union. Parecoxib is a COX2 selective inhibitor in the same category as celecoxib (Celebrex) and rofecoxib (Vioxx). As it is injectable, it can be used preoperatively when patients are unable to take oral medications. It is approved through much of Europe for short term perioperative pain control much in the same way ketorolac (Toradol) is used in the United States. A letter of non-approval for parecoxib was issued by the FDA in 2005.
Mechanism of Action:
By inhibition of cyclooxygenase-2 (COX-2)-mediated prostaglandin synthesis. Valdecoxib reduces the production of prostaglandins that are important mediators of pain and inflammation. The active ingredient in Dynastat is parecoxib (as parecoxib sodium).
Parecoxib Sodium Side effects:
Nausea, abdominal pain, headache, abdominal fullness, dizziness, back pain, fever, hypoactive bowel sounds, vomiting, tachycardia, somnolence.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.