Paclitaxel CAS NO 33069-62-4 Inquire about Paclitaxel
Tecoland supplies Paclitaxel bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Paclitaxel is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Paclitaxel?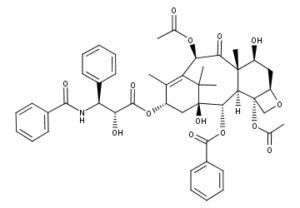
Paclitaxel is a natural substance derived from the bark of the Pacific yew tree (Taxus brevifolia) by taking a naturally present substance from the tree and chemically altering it to form the drug. The resultant drug is administered intravenously. It is used as a chemotherapy drug to treat people with a wide variety of cancers. It has been approved by the U.S. Food and Drug Administration (FDA) for treating breast and ovarian cancers as well as lung and AIDS-related cancers. Paclitaxel is a cytotoxic anticancer drug, caution should be exercised in handling paclitaxel.
Paclitaxel description
Paclitaxel is a natural product with antitumor activity. Natural Paclitaxel is extracted from Taxus chinensis and purified by HPLC method without any semi-synthesis process.
Paclitaxel is a white to off-white crystalline powder and is highly lipophilic, insoluble in water, and melts at around 216-217°C. It has a molecular weight of 853.93 and a molecular formula C47H51NO14 .
Paclitaxel introduction
Paclitaxel is chemotherapy that is given as a treatment for some types of cancer. It is most commonly used to treat ovarian, breast and non-small cell lung cancer. This section describes paclitaxel, how it is given and some of its possible side effects. It should ideally be read with Cancer BACUP’s general information on chemotherapy, which gives more information and advice.
Paclitaxel is a clear fluid. Paclitaxel may be given as an infusion (drip) into the vein through a cannula (a fine tube inserted into the vein)
Paclitaxel side effects
Each person’s reaction to chemotherapy is unique. Some people have very few side effects, while others may experience more. The side effects described in this section will not affect everyone who is given paclitaxel, and may be different if you are having more than one chemotherapy drug.
If paclitaxel solution contacts the skin, wash the skin immediately and thoroughly with soap and water. Following topical exposure, events have included tingling, burning and redness. If paclitaxel contacts mucous membranes, the membranes should be flushed thoroughly with water. Upon inhalation, dyspnea, chest pain, burning eyes, some throat and nausea have been reported.
Paclitaxel and aching or pain in joints and muscles
This may occur a few days after paclitaxel is given. It does not usually last long and your doctor may prescribe painkillers or anti-inflammatory drugs to help.
Paclitaxel and skin changes
Paclitaxel can cause a rash, which may be itchy. Your doctor can prescribe medicine to help with this.
Paclitaxel and numbness or tingling in hands or feet
This is due to the effect of paclitaxel on nerves and is known as peripheral neuropathy. You may also notice that you have difficulty doing up buttons or similar fiddly tasks. Tell your doctor if you notice any numbness or tingling in your hands or feet. This usually improves slowly a few months after the treatment is finished.
Paclitaxel and headaches
Some people find that paclitaxel causes headaches. Let your doctor know if you have headaches while having treatment with paclitaxel.
You will be given steroid tablets (usually dexamethasone) to take before the paclitaxel to reduce the chance of an allergic reaction. If you are given any of these tablets before treatment it is important to take them as directed and to tell your doctor or if you forget.
Paclitaxel and changes in heart rate
Paclitaxel can sometimes cause a temporary slowing of the heart rate known as bradycardia. This usually does not cause any harm.
Paclitaxel may be temporarily affect liver
Paclitaxel may cause changes in the way that your liver works, which return to normal when the treatment is finished. This is very unlikely to cause you any harm, but your doctor will monitor this carefully. Samples of your blood will be taken from time to time to check your liver function.
Paclitaxel additional information:
While paclitaxel is being given, it can cause pain at the place where the injection is given, or along the vein. If you feel pain, tell your doctor or . They can slow the drip down to reduce the feeling.
Paclitaxel contraception:
It is not advisable to become pregnant or father a child while taking paclitaxel as it may harm the developing foetus. It is important to use effective contraception whilst taking this drug, and for at least a few months afterwards. Again, discuss this with your doctor.
Paclitaxel background and applications
Paclitaxel may be one of the most important anticancer agents to be developed over the pasttwo decades. With its unique mechanism of action as an inducer of tubulin assembly,paclitaxel has demonstrated impressive antitumor activity in patients with breast, lung (both non-small cell and small cell), head and neck, and advanced and platinum-refractory ovarian carcinomas.
Paclitaxel’s antitumor activity was discovered in the 1960s during a largescale 35,000 plants-screening program sponsored by the National Cancer Institute (NCI), USA.
In 1983, NCI began conducting clinical trials of paclitaxel’s safety and its effectiveness against various types of cancer. Demand for paclitaxel increased in 1989 after the investigators at The Johns Hopkins Oncology Center reported that the drug produced partial or complete responses (shrinking or disappearance of the tumor) in 30 percent of previously treatedpatients with advanced ovarian cancer. it was clear that ovarian cancer patients with few otheroptions benefited from the treatment.
In December 1992, FDA approved the use of paclitaxel for refractory (treatment-resistant) ovarian cancer Subsequently, clinical trials using paclitaxel for the treatment of advanced breast cancer demonstrated that the drug is effective against this disease.
In April 1994, FDA approved the use of paclitaxel for breast cancer that has recurred within 6 months after the completion of initial chemotherapy and for metastatic breast cancer that is not responding to combination chemotherapy.
March 1997, FDA designated Taxol (Paclitaxel Injection) as Orphan Drug for treatment of AIDS-related Kaposi’s sarcoma.
April 1998, FDA gave an additional approval for Paclitaxel injections, for first-line therapy for the treatment of advanced carcinoma of the ovary in combination with cisplatin.
Researchers continue to look for new and better ways to use paclitaxel in the treatment of cancer. They are studying paclitaxel’s effectiveness when used to treat breast and ovarian cancer earlier in the course of these diseases and when used in combination with other drugs. Trials to test the effectiveness of paclitaxel against many other types of cancer, including leukemia, lymphoma, and cancers of the lung, head and neck, and colon, also are in progress. In addition, researchers are investigating ways to overcome some patients’ resistance to paclitaxel and are trying to develop methods for using the drug in patients who have impaired organ function
To date, 180 clinical trials have been conducted, researchers have found the drug to be active in lung, head and neck, bladder, esophageal, and germ cell cancers.
Paclitaxel Supply resources
Early research using paclitaxel was limited by a restricted supply due to several difficulties in obtaining the drug from the Taxus brevifolia (Pacific yew). The concentration of the compound in yew bark is low, and paclitaxel extraction is complex and expensive. The Taxus brevifolia (Pacific yew) is a limited resource, it grows very slow and is located in old-growth forests that are the habitat of the endangered spotted owl.
As demand for paclitaxel increased, researchers have been exploring new resources to increase the availability of paclitaxel.
BMS produces Paxlitaxel Injection (Taxol) via a semi-synthesis process from Taxus baccata (Europe Yew).
There have been many research reports on the constitutions of othet yew tree species worldwide.
Scientists in US, China, Japan, Taiwan and other countries have done detailed research on Taxus chinensisi (Chinese yew) as the new resource of paclitaxel. There are four Chinese yew species growing on the territory of China. Scientists proved that all of them contain paclitaxel at a similar level as the Taxus brevifolia. Scientists have isolated and identified 110 taxanes from Chinese yews, and 36 of them were new taxanes. in vitro and in vivo studies proved that paclitaxel has the strongest anti-cancer activity.
Chinese pharmaceutical enterprises in cooperate with Chinese academic institutions developed a new production line to extract pure paclitaxel from planted 3-5 years young Taxus chinensis. With the large field of planted young Taxus chinensis, which contains paclitaxel as high as 0.015 – 0.02%, and the new manufacturing technology, China’s Natural Paclitaxel has become the most reliable new resource of high quality natural paclitaxel with very competitive cost.
HPCI paclitaxel (natural) contains 99.5% pure paclitaxel, the highest quality level, has filed USA Drug Master File (DMF) and supplying USA and European Pharmaceutical companies.
Clinical trials have proved the effectuveness and safety of Natural Paclitaxel Injection (which contains paclitaxel extracted from Taxus chinensis) in cancer treatment.
The pure natural paclitaxel is ideal for the paharmaceutical manufacturers and research laboratories to use for developing new anti-cancer preparations, and new taxane derivatives.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.