Minocycline HCl CAS NO 13614-98-7 Inquire about Minocycline HCl
Tecoland supplies Minocycline HCl bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Minocycline HCl is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Minocycline HCl?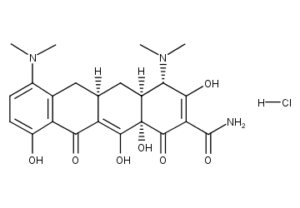
MINOCIN minocycline hydrochloride, is a semisynthetic derivative of tetracycline, 4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride. MINOCIN(minocycline) Pellet-Filled Capsules for oral administration contain pellets of minocycline HCl equivalent to 50 mg or 100 mg of minocycline in microcrystalline cellulose. The capsule shells contain the following inactive ingredients: Blue 1, Gelatin, Titanium Dioxide and Yellow 10. The 50 mg capsule shells also contain Black and Yellow Iron Oxides.
What are the possible side effects of minocycline (Dynacin, Minocin, Minocin PAC, Myrac, Solodyn)?
Get emergency medical help if you have any of these signs of an allergic reaction: hives; difficulty breathing; swelling of your face, lips, tongue, or throat.
Stop using minocycline and call your doctor at once if you have any of these serious side effects:
- severe headache, dizziness, blurred vision
- fever, chills, body aches, flu symptoms
- severe blistering, peeling, and red skin rash
- urinating less than usual or not at all
- pale
What are the precautions when taking minocycline (Minocin Capsules)?
Before using minocycline, tell your doctor or pharmacist if you are allergic to it; or to other tetracycline medication (such as doxycycline, tetracycline); or if you have any other allergies. This product may contain inactive ingredients, which can cause allergic reactions or other problems. Talk to your pharmacist for more details.
Before using this medication, tell your doctor or pharmacist your medical history, especially of: kidney disease, liver disease.
This drug may make you dizzy or drowsy. Do not drive, use machinery, or do any activity that requires alertness until you are sure you can perform such activities safely. Limit alcoholic beverages.
This medication may rarely make you more sensitive to the sun. Avoid prolonged sun exposure, tanning booths, and…
DOSAGE AND ADMINISTRATION
THE USUAL DOSAGE AND FREQUENCY OF ADMINISTRATION OF MINOCYCLINE DIFFERS FROM THAT OF THE OTHER TETRACYCLINES. EXCEEDING THE RECOMMENDED DOSAGE MAY RESULT IN AN INCREASED INCIDENCE OF SIDE EFFECTS.
MINOCIN?(minocycline) Pellet-Filled Capsules Capsules may be taken with or without food (See CLINICAL PHARMACOLOGY.)
Ingestion of adequate amounts of fluids along with capsule and tablet forms of drugs in the tetracycline-class is recommended to reduce the risk of esophageal irritation and ulceration. The pellet-filled capsules should be swallowed whole.
For Pediatric Patients Above 8 Years Of Age
Usual pediatric dose: 4 mg/kg initially followed by 2 mg/kg every 12 hours, not to exceed the usual adult dose.
Adults
The usual dosage of MINOCIN?(minocycline) Pellet-Filled Capsules is 200 mg initially followed by 100 mg every 12 hours. Alternatively, if more frequent doses are preferred, two or four 50 mg pellet-filled capsules may be given initially followed by one 50 mg capsule 4 times daily.
Uncomplicated gonococcal infections other than urethritis and anorectal infections in men: 200 mg initially, followed by 100 mg every 12 hours for a minimum of 4 days, with post-therapy cultures within 2 to 3 days.
In the treatment of uncomplicated gonococcal urethritis in men, 100 mg every 12 hours for 5 days is recommended.
For the treatment of syphilis, the usual dosage of minocycline hydrochloride should be administered over a period of 10 to 15 days. Close follow-up, including laboratory tests, is recommended.
In the treatment of meningococcal carrier state, the recommended dosage is 100 mg every 12 hours for 5 days.
Mycobacterium marinum infections: Although optimal doses have not been established, 100 mg every 12 hours for 6 to 8 weeks have been used successfully in a limited number of cases.
Uncomplicated urethral, endocervical, or rectal infection in adults caused by Chlamydia trachomatis or Ureaplasma urealyticum: 100 mg orally, every 12 hours for at least 7 days.
Ingestion of adequate amounts of fluids along with capsule and tablet forms of drugs in the tetracycline-class is recommended to reduce the risk of esophageal irritation and ulceration.
The pharmacokinetics of minocycline in patients with renal impairment (CLCR (80 mL/min) have not been fully characterized. Current data are insufficient to determine if a dosage adjustment is warranted. The total daily dosage should not exceed 200 mg in 24 hours. However, due to the anti-anabolic effect of tetracyclines, BUN and creatinine should be monitored.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.