Methotrexate CAS NO 59-05-2 Inquire about Methotrexate
Tecoland supplies Methotrexate bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Methotrexate is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Methotrexate?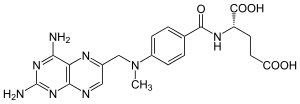
Methotrexate (rINN), abbreviated MTX and formerly known as amethopterin, is an antimetabolite and antifolate drug. It is used in treatment of cancer, autoimmune diseases, ectopic pregnancy, and for the induction of medical abortions. It acts by inhibiting the metabolism of folic acid. Methotrexate began to replace the more toxic antifolate aminopterin starting in the 1950s. The drug was developed by Yellapragada Subbarao.
What is the mechanism of action?
Methotrexate anti-tumor activity is a result of the inhibition of folic acid reductase, leading to inhibition of DNA synthesis and inhibition of cellular replication. The mechanism involved in its activity against rheumatoid arthritis is not known.
How to dose Methotrexate?
For psoriasis or rheumatoid arthritis, methotrexate is usually taken once a week, either as a single dose or split up into three doses taken every 12 hours. For adults with rheumatoid arthritis, a typical oral dosage is methotrexate 7.5 mg once weekly or 2.5 mg every 12 hours for three doses (once weekly).
For adults with psoriasis, a typical oral methotrexate dose is 10 to 25 mg once weekly or 2.5 mg every 12 hours for three doses (once weekly).
What are the side effects?
- mouth sores
- rash
- diarrhea
- blood count abnormalities
- cirrhosis of liver (rare)
- persistent cough
- unexplained shortness of breath
- hair loss (gradual)
- sun sensitivity
Storage
Methotrexate should be stored at room temperature 15 C to 30 C (59 F to 86 F), avoiding light.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.