Macitentan CAS NO 441798-33-0 Inquire about Macitentan
Tecoland supplies Macitentan bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Macitentan is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Macitentan?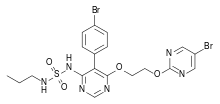
Macitentan (trade name Opsumit) is an endothelin receptor antagonist (ERA) approved for the treatment of pulmonary arterial hypertension (PAH). The other two ERAs marketed as of 2014 are bosentan and ambrisentan. However, macitentan has a 50-fold increased selectivity for the ETA subtype compared to the ETB subtype. The drug received approval from the U.S. Food and Drug Administration (FDA) on October 13, 2013.
Mechanism of Action:
Macitentan is an antagonist/blocker of endothelin receptors. Endothelin receptors are found in the endothelial cells of blood vessels and smooth muscle. Macitentan binds to the receptors, endothelin A and B (ETA and ETB), which prevents the agonist endothelin -1 (ET-1) from binding and stimulating the ETA and ETB receptors.
Pharmacokinetics:
Macitentan is taken as a 10 mg oral dose once a day. Its half-life in humans is about 16 hours and steady state is reached by the third day of administration. It is absorbed slowly into the plasma. Macitentan dealkylates into the active metabolite ACT-132577, which reaches its peak plasma concentration about 30 hours after the first dose is administered, and has a half-life of approximately 48 hours. Although ACT-132577 has a lower affinity for the ET receptors than its parent compound, It maintains higher plasma concentrations than macitentan. Both compounds can be excreted from the body through the urine or feces.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.