Ingenol Mebutate CAS NO 75567-37-2 Inquire about Ingenol Mebutate
Tecoland supplies Ingenol Mebutate bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Ingenol Mebutate is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Ingenol mebutate?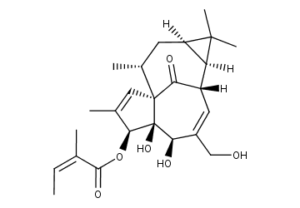
Ingenol mebutate (ingenol-3-angelate, trade name Picato) is a substance found in the sap of the plant Euphorbia peplus and an inducer of cell death. Results from four multicenter, randomized, double-blind studies have shown that ingenol mebutate gel applied topically for 2 to 3 days is effective for field treatment of actinic keratoses. The substance is an ester of a diterpene and angelic acid.
The chemical name of ingenol mebutate is:
2-Butenoic acid, 2-methyl-, (1aR,2S,5R,5aS,6S,8aS,9R,10aR)-1a,2,5,5a,6,9,10,10a-octahydro-5,5a-dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1H-2,8a-methanocyclopenta [a]cyclopropa[e]cyclodecen-6-yl ester, (2Z) – or (1aR,2S,5R,5aS,6S,8aS,9R,10aR)-5,5a-dihydroxy-4-(hydroxymethyl)-1,1,7,9-tetramethyl-11-oxo-1a, 2,5,5a,6,9,10,10a-octahydro-1H 2,8a-methanocyclopenta[a]cyclopropa[e]cyclodecen-6-yl (2Z) 2 methylbut-2-enoate.
The molecular formula is C25H34O6 and molecular weight is 430.5. Ingenol mebutate is a white to pale yellow crystalline powder.
Precautions Before Using Ingenol mebutate
Eye Exposure
Eye disorders, including severe eye pain, eyelid edema, eyelid ptosis, periorbital edema can occur after exposure. Patients should wash hands well after applying Ingenol mebutate, and avoid transfer of the drug to the periocular area during and after application. If accidental exposure occurs, the area should be flushed with water and the patient should seek medical care as soon as possible.
Local Skin Reactions
Severe skin reactions in the treated area, including erythema, crusting, swelling, vesiculation/postulation, and erosion/ulceration, can occur after topical application of Ingenol mebutate . Administration of Ingenol mebutate is not recommended until the skin is healed from any previous drug or surgical treatment.
Ingenol mebutate side effects
The most common adverse reactions (?2 %) are local skin reactions, application site pain, application site pruritus, application site irritation, application site infection, periorbital edema, nasopharyngitis and headache.
Ingenol mebutate Overdosage
Topical overdosing of ingenol mebutate could result in an increased incidence of local skin reactions.
Ingenol mebutate Storage
Store ingenol mebutate in a refrigerator at 36ºF – 46ºF (2ºC – 8ºC); excursions permitted between 32ºF – 59ºF (0ºC – 15ºC) (see USP for controlled cold temperature). Protect from freezing.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.