Ifosfamide CAS NO 3778-73-2 Inquire about Ifosfamide
Tecoland supplies Ifosfamide bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Ifosfamide is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
Ifosfamide Description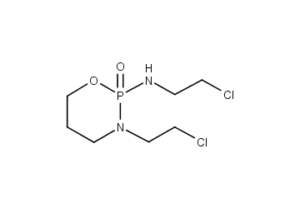
IFEX (ifosfamide for injection) single-dose vials for constitution and administration by intravenous infusion each contain 1 gram or 3 grams of sterile ifosfamide. Ifosfamide is a chemotherapeutic agent chemically related to the nitrogen mustards and a synthetic analog of cyclophosphamide. Ifosfamide is 3-(2-chloroethyl)-2-[(2-chloroethyl) amino]tetrahydro-2H-1, 3,2-oxazaphosphorine 2-oxide. The molecular formula is C7H15Cl2N2O2P and its molecular weight is 261.1. Ifosfamide is a white crystalline powder that is soluble in water.
Ifosfamide Indications
IFEX, used in combination with certain other approved antineoplastic agents, is indicated for third line chemotherapy of germ cell testicular cancer. It should ordinarily be used in combination with a prophylactic agent for hemorrhagic cystitis, such as mesna.
Ifosfamide Dosage And Administration
IFEX should be administered intravenously at a dose of 1.2 g/m2 per day for 5 consecutive days. Treatment is repeated every 3 weeks or after recovery from hematologic toxicity (Platelets ≥ 100,000/µL, WBC ≥ 4,000/µL). In order to prevent bladder toxicity, IFEX should be given with extensive hydration consisting of at least 2 liters of oral or intravenous fluid per day. A protector, such as mesna, should also be used to prevent hemorrhagic cystitis. IFEX should be administered as a slow intravenous infusion lasting a minimum of 30 minutes. Although IFEX has been administered to a small number of patients with compromised hepatic and/or renal function, studies to establish optimal dose schedules of IFEX in such patients have not been conducted.
How Supplied of Ifosfamide
- IFEX (ifosfamide for injection) is available in combination packages with the uroprotective agent Mesnex (mesna) injection or as single-dose vials as follows:
- IFEX (ifosfamide for injection)/Mesnex (mesna) injection.
- IFEX (ifosfamide for injection).
Store at controlled room temperature 20C to 25C (68F to 77F).
Protect from temperatures above 30C (86F).
Procedures for proper handling and disposal of anticancer drugs should be considered. Skin reactions associated with accidental exposure to IFEX may occur. The use of gloves is recommended. If IFEX solution contacts the skin or mucosa, immediately wash the skin thoroughly with soap and water or rinse the mucosa with copious amounts of water. Several guidelines on this subject have been published.1-7 There is no general agreement that all of the procedures recommended in the guidelines are necessary or appropriate.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.