Gemcitabine HCl CAS NO 122111-03-9 Inquire about Gemcitabine HCl
Tecoland supplies Gemcitabine HCl bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Gemcitabine HCl is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Gemcitabine HCl?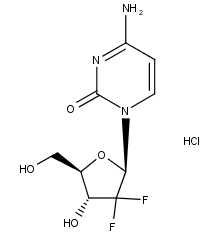
Gemcitabine is a chemotherapy drug that is given as a treatment for some types of cancer. Gemcitabine is most commonly used to treat non small cell lung cancer, pancreatic, and bladder cancer.
How does Gemcitabine HCl work?
Gemcitabine, a pyrimidine analog, is structurally similar to cytarabine, but has a wider spectrum of antitumour activity due to its different cellular pharmacology and mechanism of action.Gemcitabine is metabolized intracellularly to two active metabolites, gemcitabine diphosphate (dFdCDP) and gemcitabine triphosphate (dFdCTP). The cytotoxic effects of gemcitabine are exerted through incorporation of dFdCTP into DNA with the assistance of dFdCDP, resulting in inhibition of DNA synthesis and induction of apoptosis.Gemcitabine is a radiation-sensitizing agent.It is cell-cycle phase specific (S and G1/S-phases).
What is Gemcitabine HCl used for?
- Lung cancer, non-small cell
- Pancreatic cancer
- Bladder cancer
- Breast cancer
- Cervical cancer
- Head and neck cancer
- Lung cancer, small cell
- Lymphoma, cutaneous T-cell
- Lymphoma, Hodgkin’s disease
- Mesothelioma
- Ovarian cancer
Gemcitabine HCl dosage guideline
Refer to protocol by which patient is being treated. Numerous dosing schedules exist and depend on disease, response and concomitant therapy. Guidelines for dosing also include consideration of absolute neutrophil count (ANC). Dosage may be reduced, delayed or discontinued in patients with bone marrow depression due to cytotoxic/radiation therapy or in patients with other toxicities.
Precautions of Gemcitabine HCl
While you are being treated with gemcitabine, and after you stop treatment, do not have any immunizations (vaccinations) without your doctor’s okay. Try to avoid contact with people who have recently taken the oral polio vaccine. Check with your doctor about this.
Gemcitabine can lower your blood counts (white blood cells, red blood cells, platelets). Your doctor will check your blood counts before and after each treatment to see how it affects your blood counts. Your doctor or will give you specific instructions if your blood counts are low.
Gemcitabine can decrease your white blood cell count, especially 10 to 14 days after the drug is given. This can increase your risk of getting an infection. Report fever of 100.5F or higher, or signs of infection such as pain in passing your urine, coughing, and bringing up sputum.
Gemcitabine can decrease the platelet count. This can increase your risk of bleeding. DO NOT take any aspirin or aspirin containing medicines. Report unusual bruising, or bleeding such as nosebleeds, bleeding gums when you brush your teeth, or black, tarry stools.
Gemcitabine HCl Special Conditions To Observe
Gemcitabine may cause drowsiness. Take care if you are driving or operating machinery following this treatment.
Fertility Your ability to become pregnant or father a child may be affected by taking this drug. It is important to discuss fertility with your doctor before starting treatment.
Contraception It is not advisable to become pregnant or father a child while taking gemcitabine as it may harm the developing foetus. It is important to use effective contraception whilst taking this drug, and for at least a few months afterwards. Again, discuss this with your doctor.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.