Doripenem CAS NO 148016-81-3 Inquire about Doripenem
Tecoland supplies Doripenem bulk active pharmaceutical ingredient (API) to the pharmaceutical industry. Our Doripenem is manufactured by cGMP compliant facility. Welcome to contact us for further details including current DMF status for the product and up to date regulatory status of the manufacturing facility. We look forward to assisting you with your research and development projects.
What is Doripenem?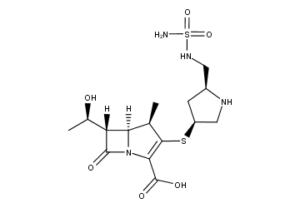
Doripenem (common name doripenem monohydrate) is an ultra-broad spectrum injectable antibiotic. It is a beta-lactam and belongs to the subgroup of carbapenems. It was launched by Shionogi Co. of Japan under the brand name Finibax in 2005 and is being marketed outside Japan by Johnson & Johnson. It is particularly active against Pseudomonas aeruginosa .
What is the mechanism of action?
Doripenem belongs to the carbapenem class of antimicrobials. Doripenem exerts its bactericidal activity by inhibiting bacterial cell wall biosynthesis. Doripenem inactivates multiple essential penicillin-binding proteins (PBPs) resulting in inhibition of cell wall synthesis with subsequent cell death. In E. coli and P. aeruginosa, doripenem binds to PBP 2, which is involved in the maintenance of cell shape, as well as to PBPs 3 and 4.
How to dose Doripenem?
The recommended dose of doripenem to treat nosocomial pneumonia in patients with augmented renal function and/or infections with pathogens with possible decreased susceptibility has been increased to 1 g every 8 hours given as a 4-hour infusion. Previous dosing regimens for doripenem in such patients were found to be insufficient.
What is the side effects?
- diarrhea that is watery or bloody;
- trouble breathing;
- pale skin, easy bruising or bleeding;
- seizure (convulsions); or
- fever, sore throat, and headache with a severe blistering, peeling, and red skin rash.
Storage
Intravenous: Reconstituted suspension may be held for 1 hr prior to dilution in infusion bag. Following dilution of the suspension with normal saline, stability is 12 hr at room temperature or 72 hr under refrigeration. Stability of solution when diluted with Dextrose 5% is 4 hr at room temperature and 24 hr under refrigeration.
Disclaimer:
Information on this page is provided for general information purposes. You should not make a clinical treatment decision based on information contained in this page without consulting other references including the package insert of the drug, textbooks and where relevant, expert opinion. We cannot be held responsible for any errors you make in administering drugs mentioned on this page, nor for use of any erroneous information contained on this page.